Question
Question: IUPAC name of the bond line structure is: 
a.) Trimethyl propane
b.) Pent-1-yne
c.) Pent-2-ene
d.) 1,3 - pentadiene
Solution
Hint: In the question structure of an organic compound is given. We start the naming by numbering and then start the naming from the substituents. And if we look at this question compound, here no substituent is present.
Complete step by step solution:
We know that IUPAC: International Union of Pure and Applied Chemistry
IUPAC is an acronym for International Union of Pure and Applied Chemistry, which is a globally recognized international chemistry standards organization that has named all the chemical organic substances in a systematic manner.
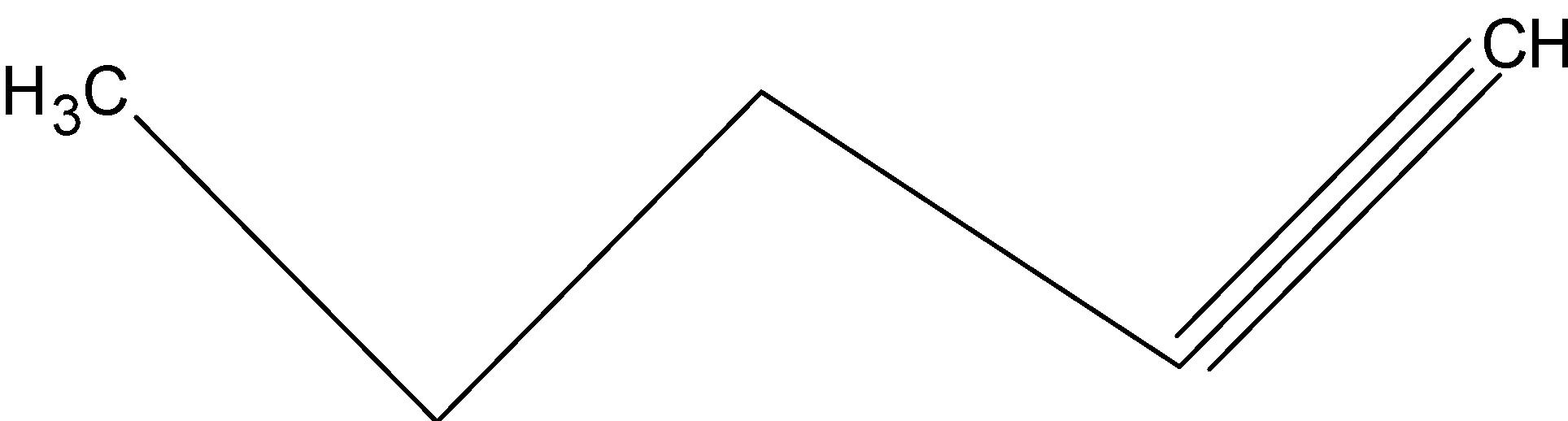
For the IUPAC naming of this compound:
First we select the main chain or parent chain which has the maximum number of carbon atoms (5 membered carbon chain in this compound),
Then we will see the number and position of substituent and do their numbering in the order of preference. Here, in this question there are no substituents.
Then we will see for the suffix and prefix, and here parent chain is a five membered chain so ‘pent’ prefix will be used and triple bond is present so ‘yne’ will be used. Now we will check the position of the triple bond, and here we will start the numbering from the end where the triple bond is present.
So, the IUPAC name of this compound is:
“pent-1-yne”
So, the correct option is “B”.
Note: In this compound there were no substituents, but if the substituents are given then we will do the numbering according to the preference of substituents or bonds or functional group.
