Question
Question: IUPAC name of \[{\text{HOOCC}}{{\text{H}}_{\text{2}}}{\text{C}}\left( {{\text{OH}}} \right)\left( {{...
IUPAC name of HOOCCH2C(OH)(COOH)CH2COOH is
(A) citric acid
(B) 2-hydroxy-1,2,3-propanetri-carboxylic acid
(C) 2-Hydroxy-1,2,3-propanoic acid
(D) hexanetrioic acid
Solution
First identify the parent chain. Then identify various substituents present. Then number the chain. Then indicate the presence of substituents by using proper locants.
Complete answer:
Let us discuss the given options:
The name 2-Hydroxy-1,2,3-propanoic acid is incorrect as it represents the presence of only one functional group.
The name hexanetrioic acid is incorrect as it represents the presence of three carboxylic groups but it does not represent the presence of hydroxyl groups.
Write the structural formula of the given compound,
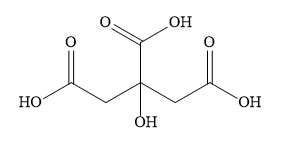
The common name is citric acid.
There are three carboxylic acid functional groups and one hydroxyl group in the given compound. You can indicate the presence of three carboxylic acid groups as a suffix of ‘tricarboxylic acid’. You can write the presence of the hydroxyl group as a prefix as ‘hydroxy’.
The parent chain contains 3 carbon atoms. This excludes the carbon atoms of carboxylic groups. You can name the parent chain as propane. You can number the parent chain from either end as it will give the same locants to substituents.
IUPAC name of HOOCCH2C(OH)(COOH)CH2COOH is 2-hydroxy-1,2,3-propanetri-carboxylic acid.
Hence, the correct option is the option (B).
Note: When the parent chains contain two (or more) carboxylic acids present at both ends of the chain, then the number of carbon atoms present in the parent chain does not include the carbon atoms of the carboxylic functional groups. Thus, in the above example, the compound is named as 2-hydroxy-1,2,3-propanetri-carboxylic acid. This compound cannot be named as 3-hydroxy-1,3,5-pentanetri-carboxylic acid.
