Question
Question: IUPAC name of neo-pentane is (A) \(2 - {\text{ethylpentane}}\) (B) \(2,2 - {\text{dimethylpentane}...
IUPAC name of neo-pentane is
(A) 2−ethylpentane (B) 2,2−dimethylpentane
(C) 2,2−dimethylpropane (D) 2−dimethylpentane
Solution
IUPAC nomenclature is a method of naming organic chemical compounds. Ideally, every possible organic compound can be named from its structural formula and vice-versa.
Complete step by step answer:
The name of compound ‘neo-pentane’ is a trivial or common name which is used to replace the IUPAC name of the compound. However, the prefix ‘neo’ is used when the two carbons form a continuous chain and these two carbons also do are the part of a termination tert-butyl group (tert refers to tertiary which means three atoms). The tert-butyl group is shown below:
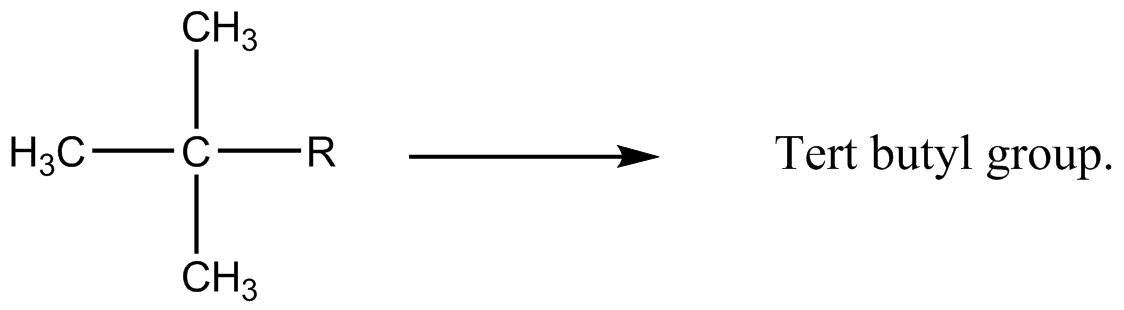
The word ‘pentane’ in the given common name of compound suggests that there are a total five carbon atoms in the given compound as the word ‘pent’ does refer to the number five. So, form these two points, the chemical structure having neo-pentane as its common name, can be concluded as:
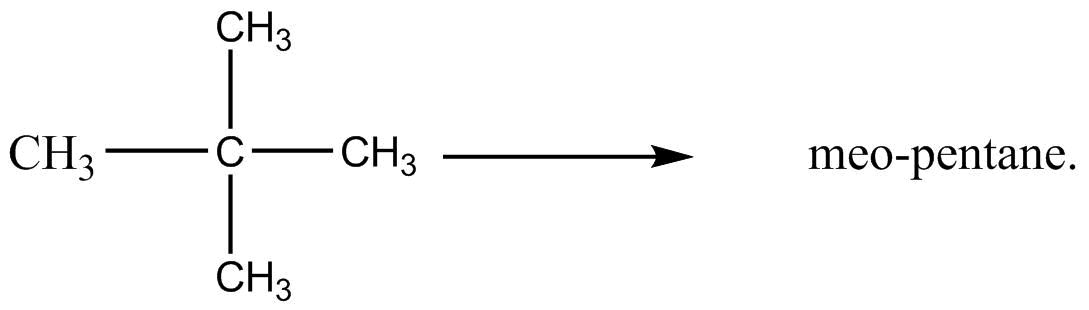
Now, for the IUPAC naming of the compound, firstly, we need to determine the longest possible straight carbon chain. In the given compound, the possible longest straight chain can be of three carbon atoms. We can see from the above structure of neo-pentane that two methyl groups are attached on the second carbon atom from the straight chain of three carbon atoms.
So, the IUPAC name of neo-pentane is: 2,2−dimethylpropane
Here, the number 2,2 represents that both the methyl groups, which are shown by the word ′di′ are attached on the second carbon atom of the chain and the word ′propane′ represents that the straight chain contains three carbon atoms.
Hence, the answer is option (C).
Note:
IUPAC stands for international union of pure and applied chemistry. The IUPAC states different rules for the naming of organic and inorganic compounds.
