Question
Question: Isostructural species are those which have the same shape and hybridization. Among the given species...
Isostructural species are those which have the same shape and hybridization. Among the given species, identify the isostructural pairs.
a.) NF3and BF3
b.) BF4−and NH4+
c.) BCl3and BrCl3
d.) NH3and NO3−
Solution
Hint : For finding isostructural species we have to find hybridization of every species of central atom then we will draw the structure of given species and find the name of their structure.
Complete step by step solution :
Isostructural chemical compounds have similar chemical structures.
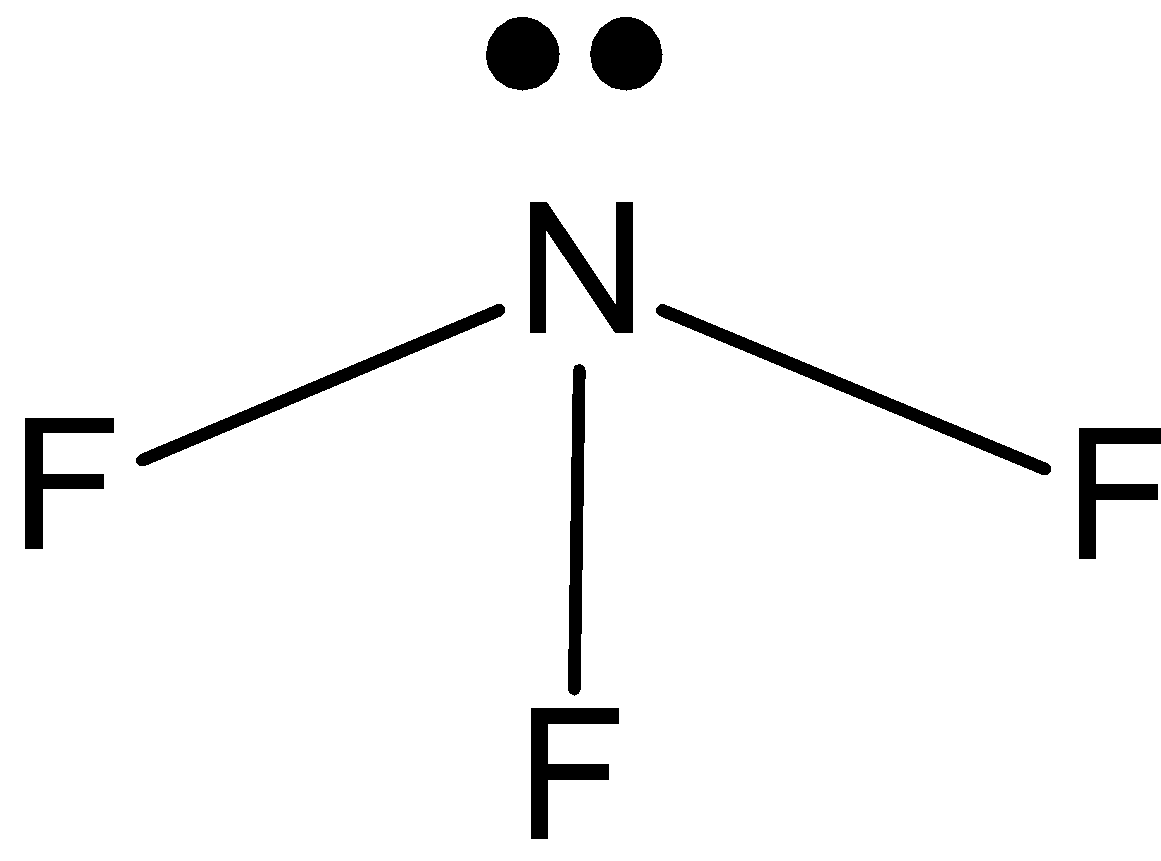
NF3: In case of NF3, we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with fluorine, NF3 has one lone pair and three bond pairs. So, the hybridization is sp3. And the shape is pyramidal.
BF3: In case of BF3, we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with fluorine, BF3 has only three bond pairs. So, the hybridization issp2. And the shape is triangular.
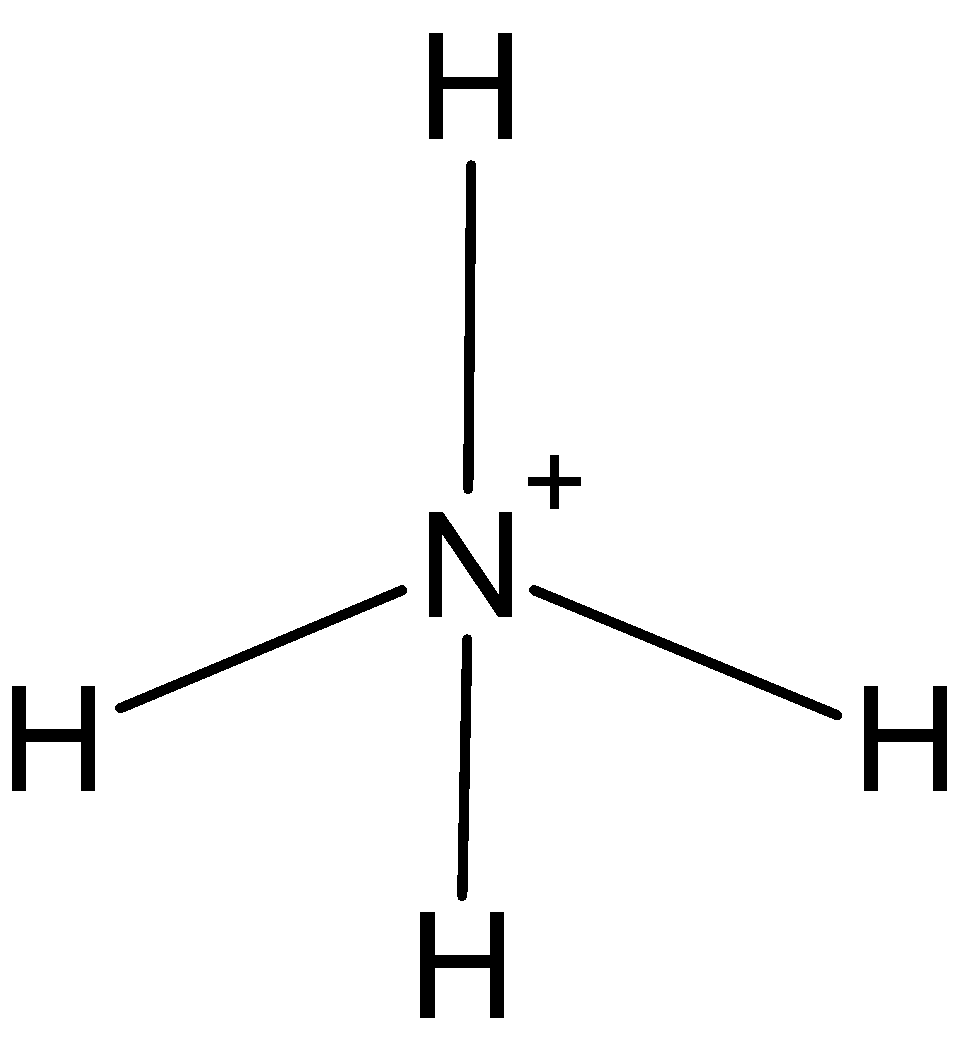
NH4+: In case ofNH4+, we know that nitrogen has five valence electrons in its outer shell. One positive charge is on it so it is making 4 bonds with hydrogen, NH4+ has only four bond pairs. So, the hybridization is sp3. And the shape is tetrahedral.
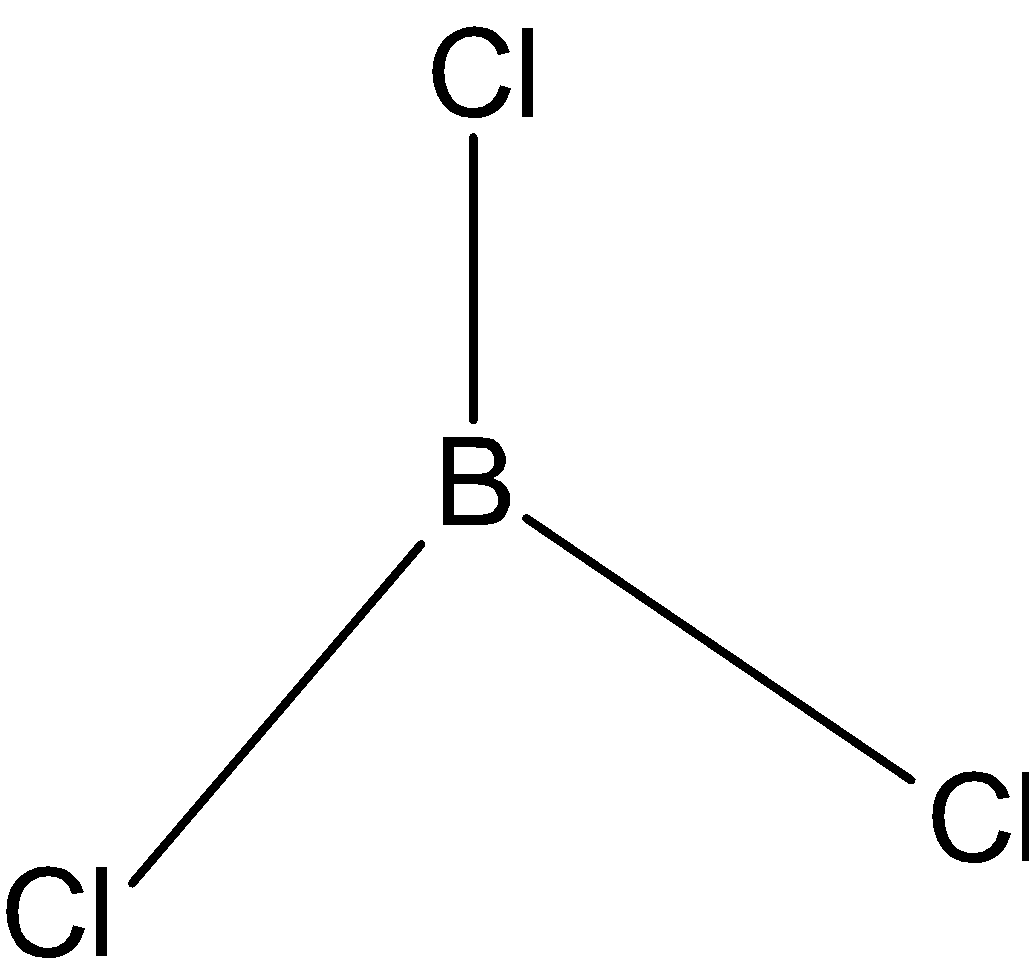
BCl3: In case of BCl3, we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with chlorine, BCl3 has only three bond pairs. So, the hybridization issp2. And the shape is triangular.
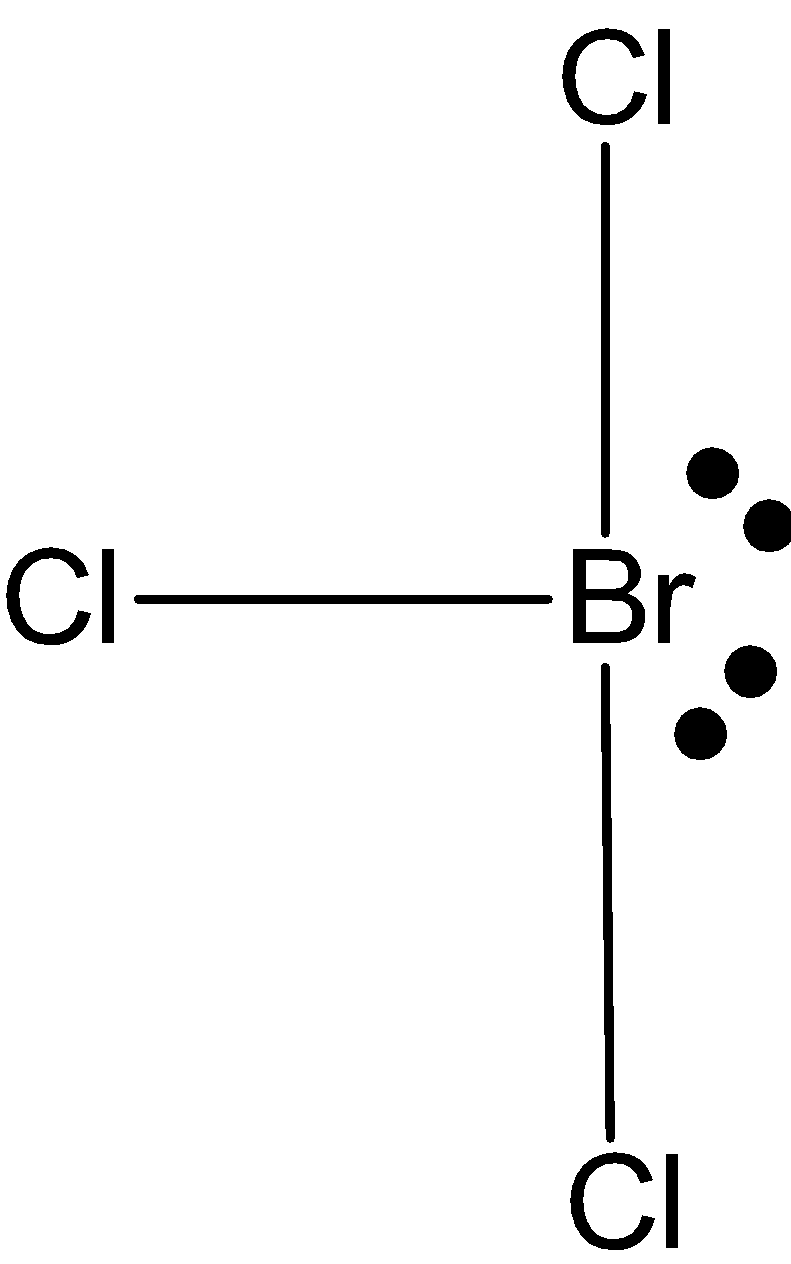
BrCl3: In case ofBrCl3, we know that bromine has seven valence electrons in its outer shell. And it is making 3 bonds with chlorine, BrCl3 has only three bond pairs and rest two lone pairs. So, the hybridization issp3d. But the shape is T-shape.
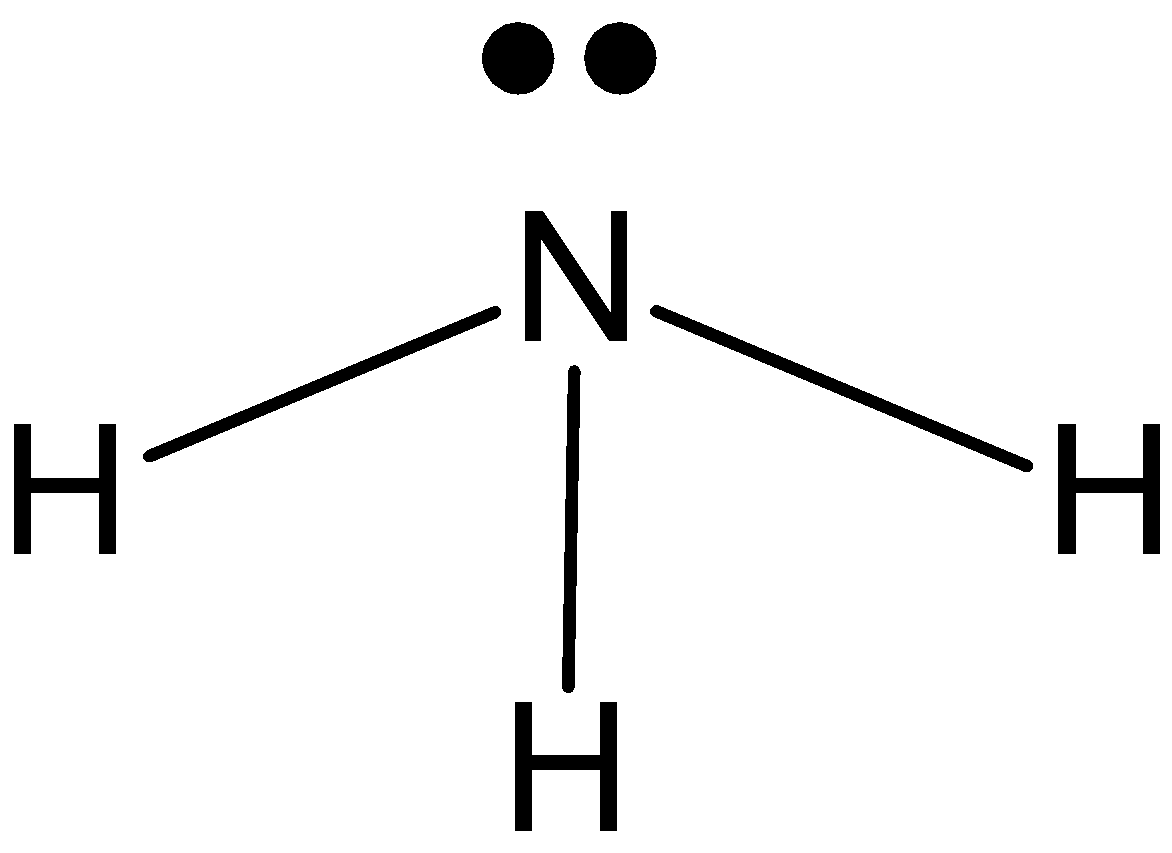
NH3: In case ofNH3, we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with hydrogen, NH3 has one lone pair and three bond pairs. So, the hybridization issp3. And the shape is pyramidal.
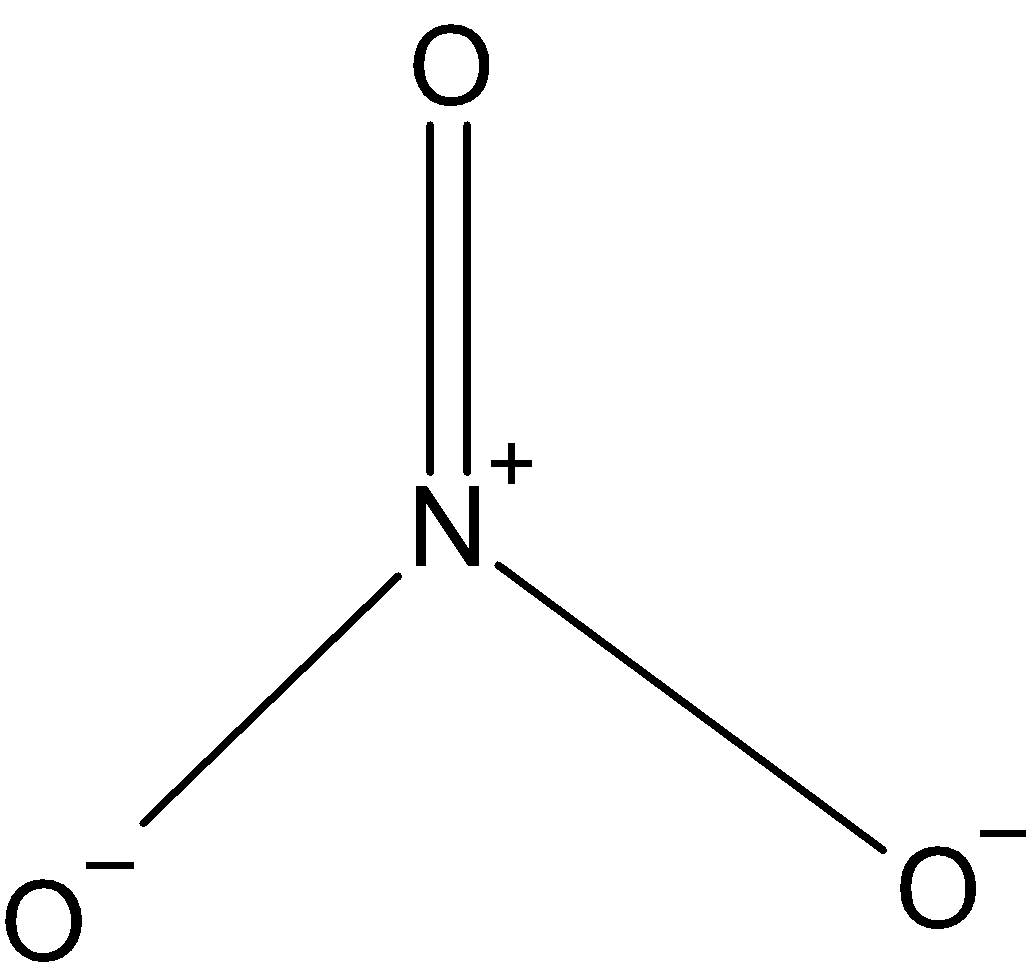
NO3−: In case ofNO3−, we know that nitrogen has five valence electrons in its outer shell. And it has one negative charge and it is making 4 bonds with oxygen, NO3− is making one double bond with single oxygen. So, the hybridization issp2. And the shape is triangular.
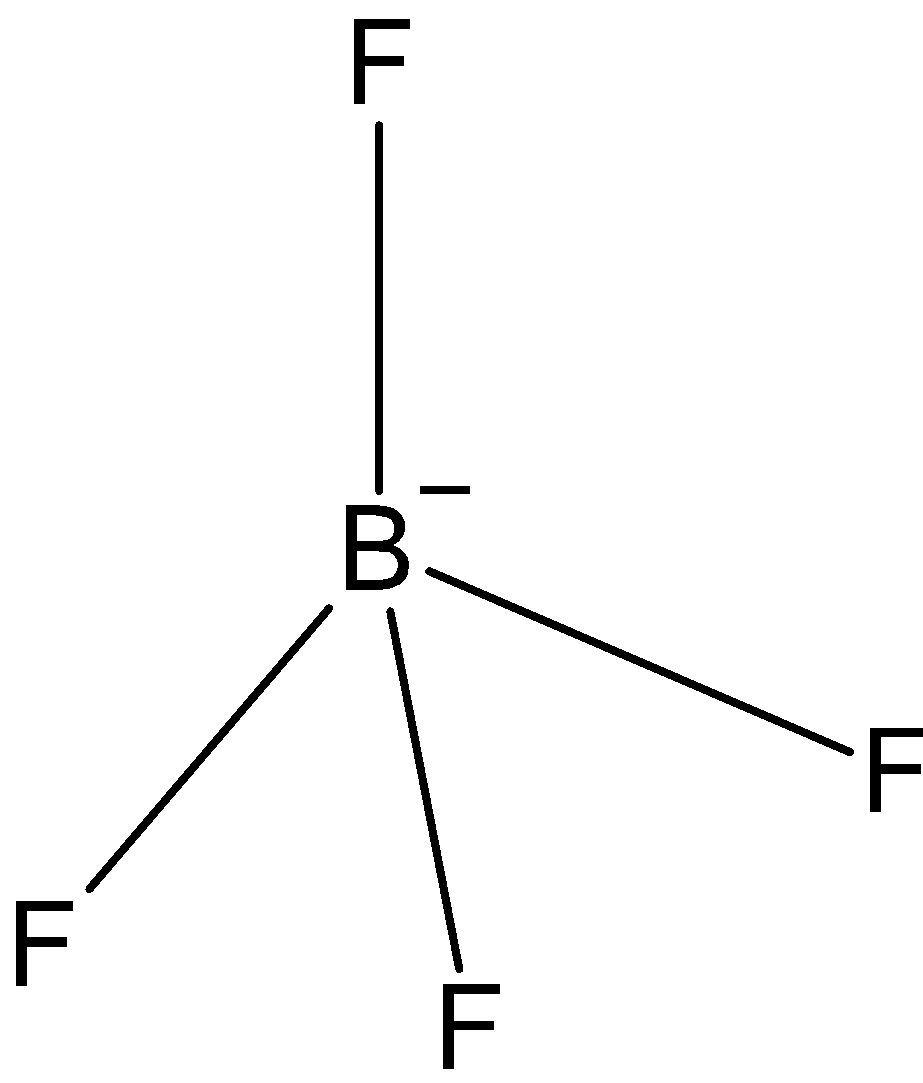
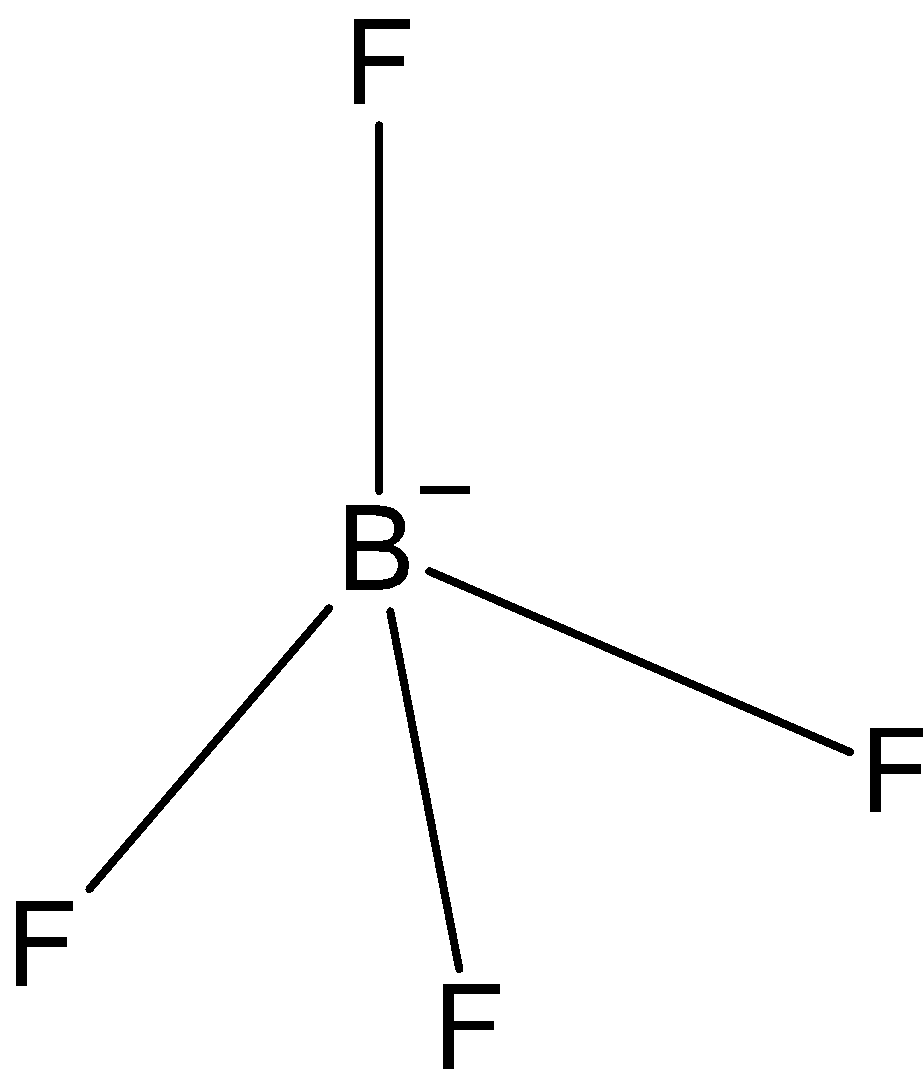
BF4−: In case of BF4−, we know that boron has three valence electrons in its outer shell. And one negative charge is on it, so it is making 4 bonds with fluorine, BF4− has only three bond pairs. So, the hybridization issp3. And the shape is tetrahedral.
So, the correct answer is “Option B”.
Note : Here should know that in case of lone pairs we cannot predict the shape according to the hybridization, for this we have to draw the structures. And according to every hybridization you have to remember their name.
