Question
Question: Is \(Z{{n}^{2+}}\) Paramagnetic or Diamagnetic?...
Is Zn2+ Paramagnetic or Diamagnetic?
Solution
Paramagnetic and Diamagnetic are the characteristics of the compound which tell the magnetic effect of the compound. If there is an unpaired electron in the compound then it will behave paramagnetically, else it will be diamagnetic. Zn2+ means there will be a loss of 2 electrons from a total number of electrons in it.
Complete step-by-step answer: The magnetic behavior of the compound tells how the compound will behave in the external magnetic field. Paramagnetic and Diamagnetic are the characteristics of the compound which tell the magnetic effect of the compound. We can tell whether the compound or element is paramagnetic or diamagnetic by observing the number of electrons in it.
If there is an unpaired electron in the compound then it will behave paramagnetically, else it will be diamagnetic.
Zinc (Zn) is the element of group 12 in the d-block and the period is 4, so its atomic number will be 30. Therefore, there are 30 electrons in the zinc. The electronic configuration will be:
1s22s22p63s23p64s23d10
Now, the given form of zinc Zn2+ means there will be a loss of 2 electrons from the total number of electrons in it. So, the number of electrons will be 28. The electronic configuration will be:
1s22s22p63s23p63d10
So, let us see the arrangement of electrons in 3d:
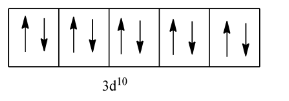
As we can see that there is no unpaired electron in this element, so it will be a diamagnetic element.
Therefore, Zn2+ is a diamagnetic element.
Note: Diamagnetic means that when the zinc is placed in the external magnetic field, it will be weakly magnetized, and paramagnetic means that when the compound is placed in the external magnetic field, it will be strongly magnetized.
