Question
Question: Is water an organic compound?...
Is water an organic compound?
Solution
Water is defined as a chemical substance that is present in solid phase, liquid phase and gaseous phase. It consists of hydrogen and oxygen elements. It is an essential compound which is tasteless and odourless. It is in liquid form at room temperature.
Complete step-by-step answer:
Let us discuss some properties of water-
-Water is a polar molecule which has partial positive charge on hydrogen and partial negative charge on oxygen. The structure of water molecules is bent because oxygen is an electronegative atom that attracts electrons.
-Water has the capability to dissolve many ionic and polar substances. Therefore, it is considered as an excellent solvent.
-The heat capacity of water is very high because it takes a lot of energy to raise the temperature of water.
-Water has the ability to form hydrogen bonds with one another. Therefore, water molecules have strong cohesive properties.
Organic compounds are defined as a compound that consists of carbon- hydrogen bonds. There are large numbers of organic compounds present. Water is not an organic compound because it does not contain carbon atoms that are covalently bonded with hydrogen atoms. It is grouped under an inorganic compound. For a molecule to be considered as an organic compound, it must contain one carbon atom in the molecule. If we see the structure of H2O, we can straight away notice that there is no carbon in the structure. Therefore, we conclude that water is not an organic compound.
Let us discuss the structure of water molecule-
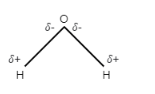
Note: It is to note that water is not a universal solvent that means it does not dissolve everything. Water has the ability to dissolve only ionic and polar substances like sugar. Non polar compounds do not contain partial positive charge and partial negative charge. Therefore, these non polar substances are not able to attract water molecules. For example- oil.
