Question
Question: Is toluene saturated, unsaturated or aromatic?...
Is toluene saturated, unsaturated or aromatic?
Solution
Saturated compounds are those which have a single bond between two carbon atoms. Unsaturated compounds are those which have either double or triple bond between two carbon atoms. Aromatic compounds are generally cyclic compounds which give aroma on its own.
Complete Answer:
⇒ Saturated compounds are those hydrocarbons which do not have any double or triple bond in their structure. Such compounds are the simplest form of hydrocarbons also. They have a general formula CnH2n+2 , where n is greater than or equal to one.
⇒ Unsaturated compounds are those which have either double or triple bond in its structure between the two carbon atoms. These compounds are further divided into alkenes and alkynes. They might have a general formula CnH2n or CnH2n−2 where n is greater than one.
⇒ Aromatic compounds are those which have ring structure and are planar in structure. Aromatic compounds produce aroma (smell) on their own. These compounds also follow Huckel rule of aromaticity according to which it must have (4n+2) pi electrons, where n can take values from zero.
The structure of toluene can be represented as:
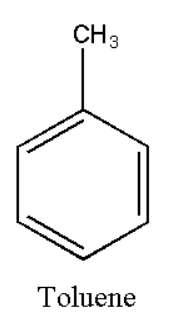
Based on the above definitions it can be classified as an unsaturated and aromatic compound.
Note:
Toluene contains six pi electrons which constitute three double bonds present in the structure. Thus it follows the Huckel rule for n equal to one. Toluene is a common name, its IUPAC name is methyl benzene. It must be noted that benzene rings are aromatic in nature because they follow the Huckel rule of aromaticity. Since they contain double bonds they are also called unsaturated aromatic compounds.
