Question
Question: Is this possible? If yes, then how? 
Solution
Benzene can be converted into ketone by electrophilic substitution reaction. During this reaction generation of an electrophile occurs. And the transformation of the aromatic ring into a ketone takes place.
Complete Solution :
- Benzene can be converted into ketone by Friedal craft acylation reaction.
- It is found that in Friedel-Crafts acylation reaction, the addition of an acyl group to an aromatic ring takes place. This reaction happens in presence of a Lewis acid catalyst like AlCl3.
- We can also use acid anhydride as an alternative to the acyl halide in Friedel-Crafts acylation reaction.
- Let’s discuss about the reaction that takes place between benzene and an acyl chloride:
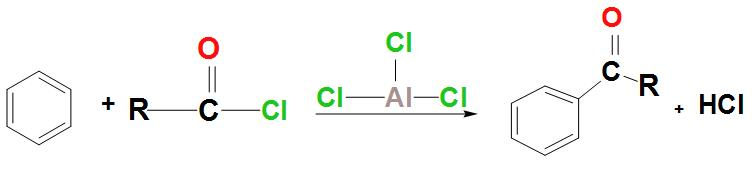
- Let’s discuss about the mechanism of Friedel-Crafts acylations
Friedel-Crafts acylations take place through a four-step mechanism.
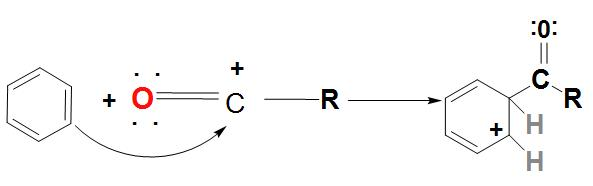
- In the first step, we can see that Lewis acid catalyst (AlCl3) and the acyl halide react with each other and an acylium ion is formed, which is stabilized by resonance.
- In the next step the acylium ion (RCO+) an electrophilic attack on the aromatic ring. As there is a complex formed, the aromaticity of the ring is found to be temporarily lost.
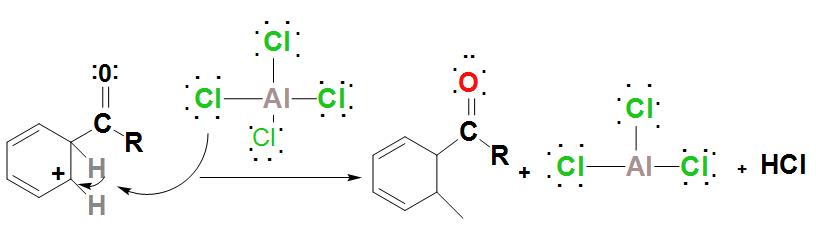
- It is found that the intermediate complex is deprotonated. The proton attaches to a chloride ion HCl and leads to regeneration of AlCl3 catalyst.
- Further, the regenerated catalyst is now found to attack the carbonyl oxygen. Hence, by adding water to the products that are formed in the above steps, the ketone product must be liberated.
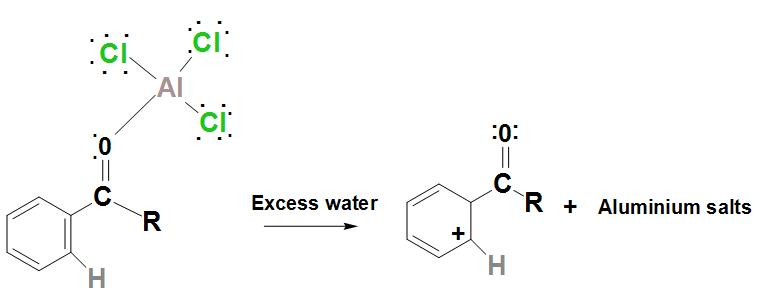
Hence, we can conclude that benzene can be converted into ketone by Friedel-Crafts acylation reaction.
Note: - Friedel-Crafts acylation has advantages over that of Friedel-Crafts alkylation. In Friedel-Crafts acylation, polyacrylate doesn’t take place as the product ketone is less reactive than that of reactant.
- Whereas, in case of Friedel-Crafts alkylation, polyalkylation takes place.
