Question
Question: Is the molecule shown below chiral or achiral? 
Solution
The molecule given to us is an allene. It belongs to the cumulenes family. It is named so because it has cumulative double bonds (consecutive double bonds). The central carbon is the only one involved in pi bonding. The hybridisation of the central carbon is sp and that of end carbons are sp2.
Complete Step By Step Answer:
From the structure of the allene it is clear that it is not a planar molecule. The two hydrogens on C-1 are planar, since they are denoted by solid single lines, and the two substituents on C-3 are one above the plane and one below the plane denoted by wedge and dash.
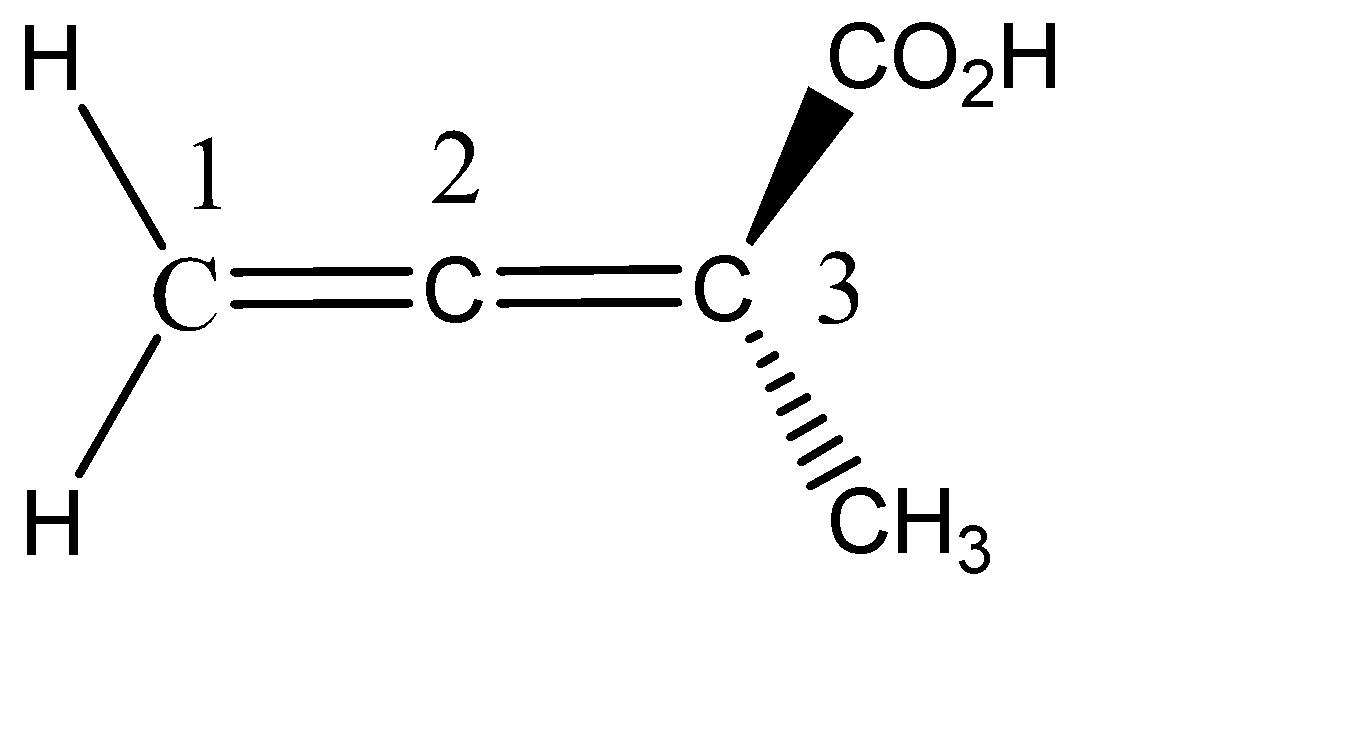
For a molecule to be chiral, the molecule should not have any plane of symmetry and any centre of symmetry. Plane of symmetry is that plane, which divides the molecule into two equal halves, and if the two halves are identical, then the molecule is symmetrical, and it becomes achiral.
Let us consider the given molecule of allene, if it has POS or COS.
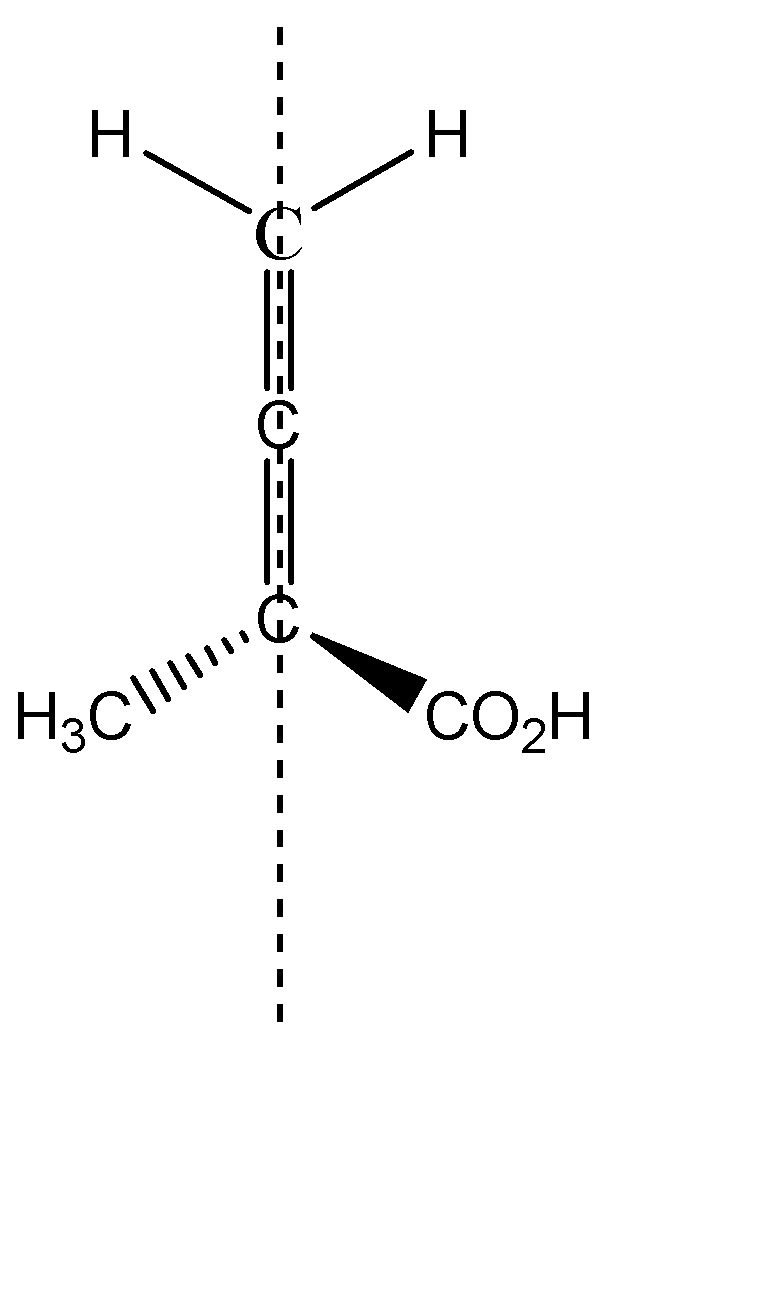
The two substituents methyl and carbonyl, being above and below the plane will be cut into equal halves, and the two hydrogens which are in plane, are cut into two halves. Hence we can see that the two halves of the molecule are mirror images of each other, therefore, we can say that a given allene has a Plane Of Symmetry and therefore, it becomes achiral.
The given molecule is achiral and this is the required answer.
Note:
A shortcut to determine the chirality in allenes is that if either of the two ends of the allene are attached to identical substituents, it will be achiral (since it will have POS) and if neither of the two ends have identical substituents it will be chiral.
