Question
Question: Is pyrene an aromatic hydrocarbon?...
Is pyrene an aromatic hydrocarbon?
Solution
As we know that aromatic hydrocarbons is a cyclic hydrocarbon with alternating double and single bonds. Aromatic hydrocarbons have (4n+2)π electrons. The most common example of aromatic hydrocarbon is benzene.
Complete answer:
We know that aromatic hydrocarbons is a cyclic hydrocarbon with alternating double and single bonds. According to Huckel’s rule of aromaticity, aromatic hydrocarbon has (4n+2)π
electrons.
In the given question, we are asked to determine whether pyrene is an aromatic hydrocarbon or not. For this, first let’s see the structure of pyrene.
Structure of pyrene-
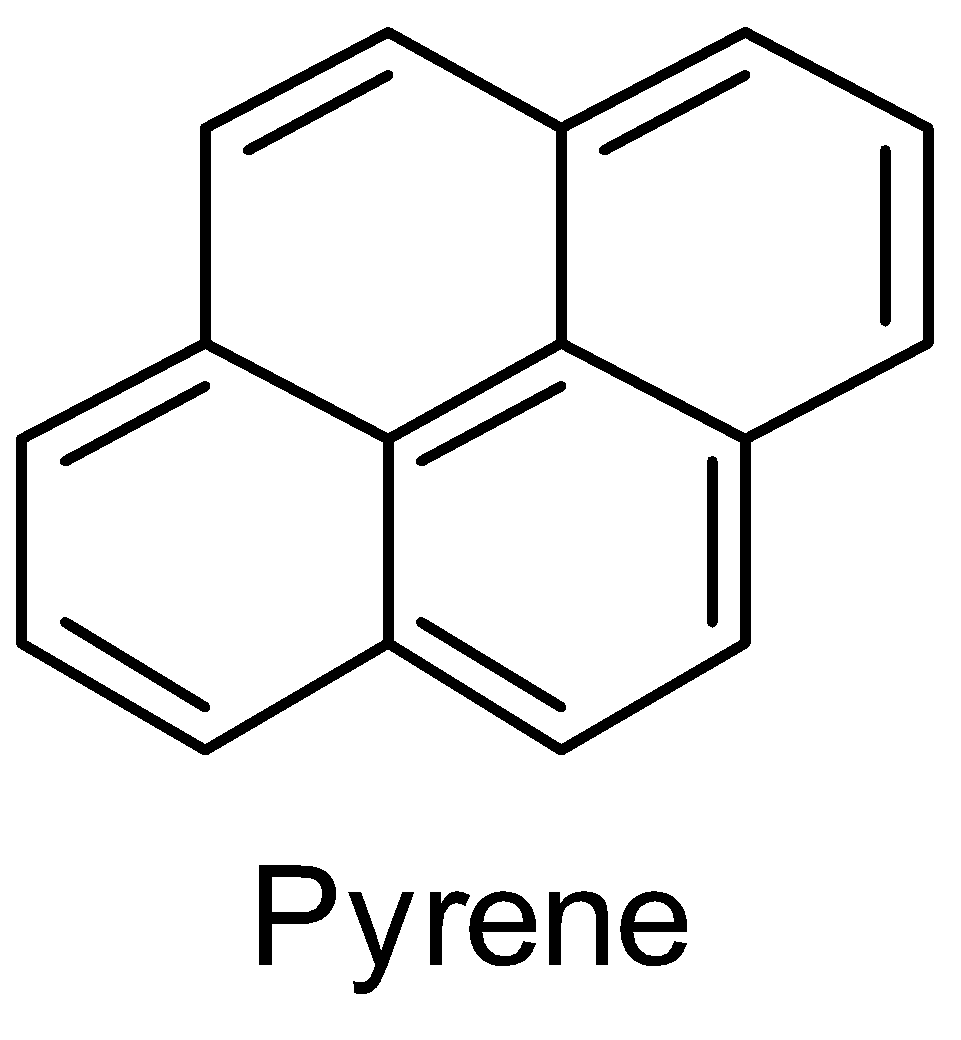
Pyrene belongs to the group of compounds called polycyclic aromatic compounds. The structure of pyrene shows three benzene rings. By drawing the resonance of the pyrene structure, the fourth ring is also a benzene ring. We can say that pyrene is a resonance hybrid with four benzene rings.
Pyrene also has (4n+2)π electrons which satisfies the Huckel’s rule for aromaticity i.e. (4n+2)π electrons where n=3 .
Therefore, pyrene is an aromatic hydrocarbon.
Additional Information:
There are some other examples of aromatic hydrocarbons such as naphthalene that has 10π electrons and anthracene which has (4n+2)π electrons. They both aromatic hydrocarbons satisfy Huckel’s rule of aromaticity.
Note:
Aromatic hydrocarbons are more stable than their non-cyclic counterparts. The carbon-carbon bonds in aromatic hydrocarbons are equivalent. The alternating double and single bonds with different lengths. They are all the same length and equivalent to each other. To show symmetry, we often use a circle in the formula of aromatic hydrocarbons to represent pi-electrons. Most of the aromatic compounds contain six carbon rings.
