Question
Question: Is polymerization endothermic or exothermic?...
Is polymerization endothermic or exothermic?
Solution
Polymerization is the chemical reaction that joins monomer molecules to form polymer chains or three-dimensional networks in polymer chemistry. There are numerous types of polymerization, and various systems exist to classify them.
Complete answer:
Polymerization can occur in chemical compounds through a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects.
Polymerization can occur in chemical compounds through a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects.
Alkenes form polymers through relatively simple radical reactions in more straightforward polymerizations; however, reactions involving substitution at a carbonyl group require a more complex synthesis due to the way reactants polymerize. Alkanes can be polymerized as well, but only with the assistance of strong acids.
Polymerization reactions are exothermic, which means they generate heat. Ideally, the total heat produced is small and harmlessly dissipates into the reaction container. However, if there is a large amount of monomer present and the reaction is strongly exothermic, the monomers may combine too quickly. As a result, excessive heat and pressure accumulate in the reaction vessel, melting the equipment or causing an explosion.
An exothermic reaction is a chemical reaction in which energy is released in the form of heat or light. These reactions are the inverse of endothermic reactions and can be expressed mathematically as follows: Products + Reactants + Energy
Following are the polymerization reaction:
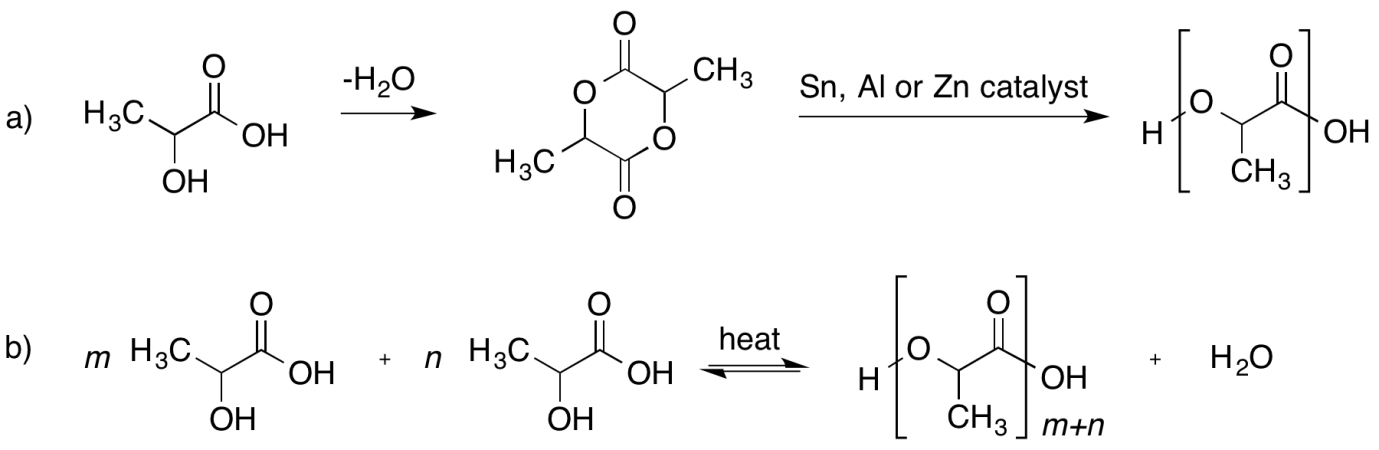
Note:
The majority of photopolymerization reactions are chain-growth polymerizations triggered by visible or ultraviolet light absorption. Light can be absorbed directly by the reactant monomer (direct photopolymerization) or indirectly by a photosensitizer, which absorbs light and then transfers energy to the monomer.
