Question
Question: Is phenolphthalein pink in acid?...
Is phenolphthalein pink in acid?
Solution
We need to know that the phenolphthalein is a chemical compound having the formula, C20H14O4 and this compound is crystalline solid and it is colorless in nature. The phenolphthalein is mainly used as an acid-base indicator. This means, we can differentiate an acid and a base compound by using this indicator. And it will show different colors in both acid and alkaline solutions.
Complete answer:
Naturally, the phenolphthalein is colorless in nature. But it turns to pink color when the base or an alkaline solution is added to it. But its color will not change in an acidic solution. This means it turns colorless in acidic solutions. Whereas, it changes to pink in basic compounds.
The phenolphthalein classifies under phthalein dyes and it can lose hydrogen ions. Hence, it is a weak acid. In the case of alkaline solution, the phenolphthalein is reacted with basic solutions and there is a formation of ions. That’s why the solution turns pink in color.
If the hydrochloric acid is added to the phenolphthalein solution, the indicator turns colorless. Because HCl is an acid. Therefore, in the acidic medium, the phenolphthalein turns colorless. Let’s see the structure of phenolphthalein,
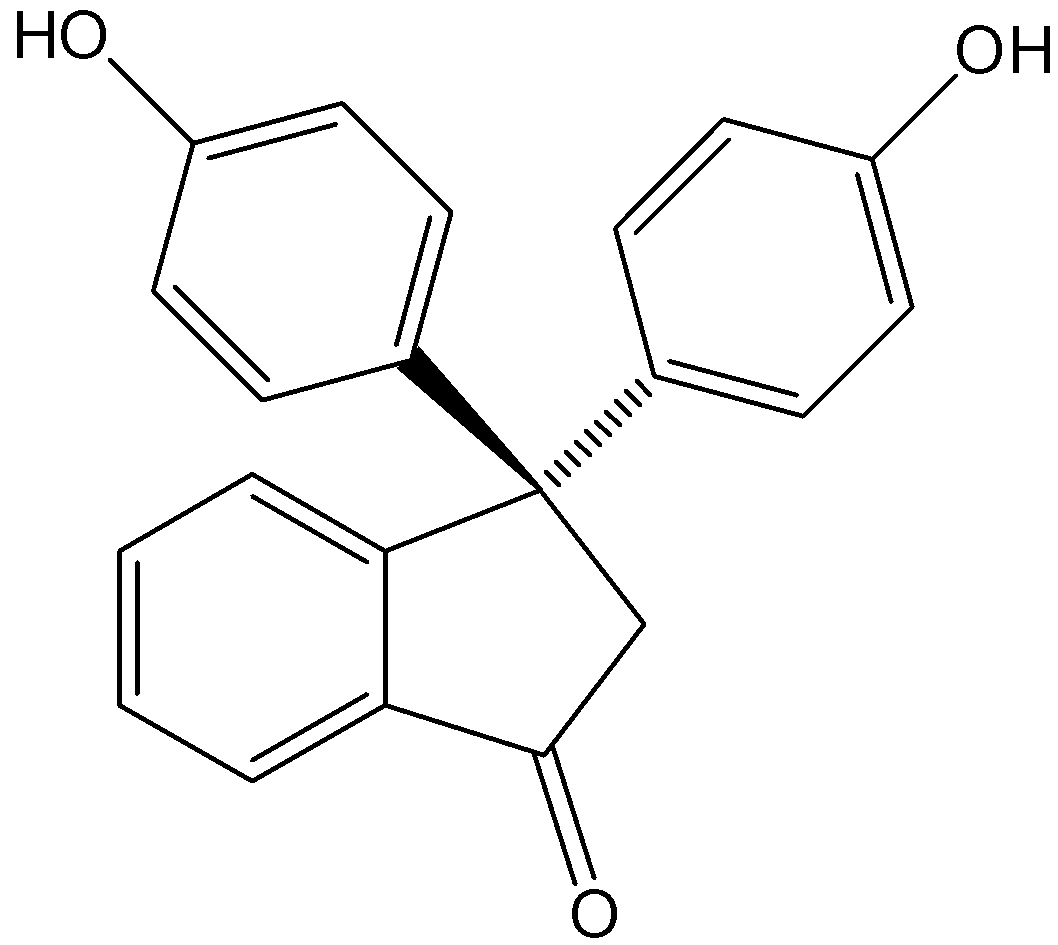
Note:
We need to know that the phenolphthalein is an acid–base indicator and it is used to check the pH of a solution. If the solution is basic, the pH of that solution should be higher than seven and the phenolphthalein turns pink in color. If the solution is acidic, the pH of the solution is less than seven and the indicator turns colorless. Therefore, in the presence of an acid, the phenolphthalein should be colorless.
