Question
Question: Is Nitrogen gas inert?...
Is Nitrogen gas inert?
Solution
Nitrogen was discovered by Rutherford and A. Lavoisier in 1772 . The atomic number assigned to the atomic nitrogen is 7 because of presences of 7 electrons in total to it. The atomic mass of the nitrogen was calculated to be 14.0067u . The nitrogen gas or dinitrogen or the N2 accounts for the 78% of the volume of earth’s atmosphere.
Complete answer:
Nitrogen is an essential element of the periodic table. It is present in minute essential organic groups like amino acids to other important day to day useful things. Though N2 accounts for the 78% of the volume of earth’s atmosphere, still the raw N2 is very rare on the earth's surface.
The electronic configuration of N is as follows: 1s2,2s22p3 . Thus, required only three more electrons to attain the nearest next noble gas configuration of Ne i.e., neon.
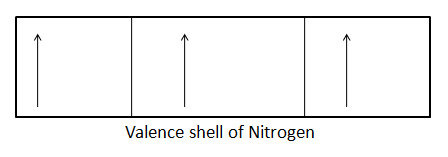
Thus by getting three more electrons, the nitrogen will be stabilized. Molecular nitrogen reacts with hydrogen to produce ammonia. It also reacts with oxygen to produce various products like dinitrogen oxide, nitrogen monoxide, dinitrogen trioxide, nitrogen dioxide, dinitrogen tetroxide, dinitrogen pentoxide. Reacts with both oxygen and hydrogen to produce nitric acid.
However if an atomic nitrogen forms a triple bond with another atomic nitrogen which then leads to dinitrogen gas or commonly called nitrogen gas. This nitrogen gas is inert because of fully filled valence shell of the nitrogen, which is as shown below
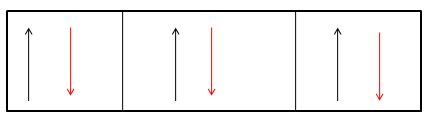
As one can see a fully filled valence shell, thus this means that the nitrogen attains the nearest noble gas configuration and attains the stability.
Note:
Dinitrogen is converted to ammonia by Haber’s process. While filling electrons to any sub shell one revise all the laws or the rules of the configuration namely aufbau rule ( electrons are filled in various subshell in the increasing order of the energy of the subshell ), hund's rule ( pairing of electrons will takes place ), pauli exclusion principle ( opposite spin of the electrons ) and the bohr bury rule.
