Question
Question: Is it true that for a value of \(n\) (n-principal quantum number), the value of \({m_l}\) (\({m_l}\)...
Is it true that for a value of n (n-principal quantum number), the value of ml (ml-magnetic quantum number) is equal to n2 ? If it is true, can you please explain this to me? Thanks.
Solution
In the atom, there are four quantum numbers which are: Principal quantum number (n), Azimuthal quantum number (l ), magnetic quantum number (ml ) and spin quantum number (ms ). Each electron of the atom has its own unique set of quantum numbers.
Complete answer:
For this we have to know about the four quantum numbers. Principal quantum number (n), is to describe the principal electron shell, i.e. the shell in which the electron is present. Azimuthal quantum number (l ), describes the shape of the orbital in which an electron is present. Magnetic quantum number (ml ) is to determine the number of orbitals and their orientation within the shell. And the spin quantum number (ms ) tells the direction of the electron spin.
The Azimuthal quantum number l depends upon the value of principal quantum n whereas ml values depend upon Azimuthal quantum number l.
The Azimuthal quantum number l can be any value ranging from 0 to n−1 when the principal quantum number n is n. So, the Azimuthal quantum number l:
l=0,1,2,......,(n−1)
The range of magnetic quantum number ml from −l to l . So, the magnetic quantum number ml:
ml=−l,....,−1,0,1,...,l
If we substitute the value of l, we can get the relation between ml and n which can be:
ml=−(n−1),...,−1,0,1,...,(n−1)
To make it more clear let’s take an example n=2 ,
for l=0 , ml=0
And for l=1, ml=−1,0,1
We can see that the values of ml is not even close to n2but the total number of ml is, if you take the values of l, is indeed equal to n2.
The magnetic quantum number tells us about the number of orbitals the subshell has. By above example, we can see if n=2 then l=0 , ml=0 indicates 2s−orbital
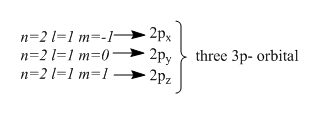
This indicates the second energy level has two subshells , one is of 2s and other one is 2p subshell. And by ml values we can get the number of orbitals each subshell has. For 2s−subshell, there is only one possible value of ml so it has one orbital. Whereas 2p−subshell there are three orbitals as it has three different values of ml. Hence the total number of orbitals in the second energy level is 4 , which equals n2 .
Note:
The only one quantum number which is not dependent on the other is spin quantum number as it indicates the direction of the electron spin. The spin of +21 is represented by ↑ , whereas the spin −21 is represented by ↓ .
