Question
Question: Is \( ICl_2^ - \) nonpolar or polar ?...
Is ICl2− nonpolar or polar ?
Solution
A structure can be defined using VSEPR Theory. The basic geometry of ICl2− is Trigonal Bipyramidal. It has 2 bond pairs, 3 lone pair electrons. The lone pairs are at an equatorial position in order to minimize the repulsion and the geometry turns out to be linear. The Cl-I-Cl are in a straight line i.e. in the Z-plane.
Complete Step By Step Answer:
In polar molecules, the charges are distributed unevenly across the bonds. Because of the opposing charges, a polar molecule has a net dipole. Polar molecules are generally hydrophilic and soluble in polar solvents.
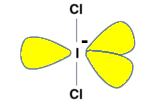
ICl2− is a nonpolar molecule. ICl2− with 22 valence electrons has 3 lone pairs and 2 shared pairs of the central I atom and the linear shape is non-polar because the dipoles cancel out each other giving net dipole moment zero. In all directions as the 3 lone pairs of electrons are 120∘ apart from the adjacent one, in a rotationally symmetric configuration, and hence the lone pair and bonding repulsion cancel out each other.
Note:
VSEPR (Valence shell electron pair repulsion theory) is also named the Gillespie –Nyholm theory. From the number of electron pairs surrounding the central atoms, the geometry of an individual molecule is predicted. The central atom is bonded to 2 or more other atoms whereas only one other atom is bonded to the terminal atom.
The central atom’s steric number is the sum of the number of atoms bonded to the central atom and the number of lone pairs set up by the non-bonding valence electron.
