Question
Question: Is Hydroboration syn or anti....
Is Hydroboration syn or anti.
Solution
Hydroboration refers to the addition of borane into alkene molecules. The chemical formula of Borane is BH3which is an electron deficient species and generally exists as a dimer diborane B2H6. Hydroboration is a concerted reaction which occurs via a four membered transition state.
Complete answer:
Hydroboration is an example of electrophilic addition reaction of alkenes. Alkenes contain double bonds and hence act as nucleophilic partners while borane is an electron deficient species and hence acts as an electrophile partner. The hydroboration of general alkene is represented by the following mechanism:
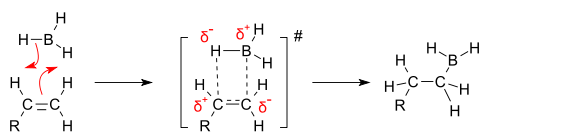
From the above diagram, we conclude that the addition of Hand BH2to alkenes takes place from the same face. Hence, addition of BH3 is a syn addition. Hydroboration is a single step reaction (concerted one) which occurs through four membered transition states. Hydroboration doesn’t stop at this stage and reacts with two more molecules of alkenes and ultimately gives a trialkyl borane in which all the three hydrogen atoms of BH3 are replaced with an alkyl group. This is illustrated by the following diagram:

Additional Information:
As hydroboration is a syn addition, therefore this reaction is an example of stereospecific reaction. Generally, hydroboration followed by oxidation is used for converting the alkenes into alcohols. The final product alcohol is formed in accordance with anti-Markovnikov's rule. Diborane instantly ignites in air that’s why it is commercially available in ether as solvent because of complex formation. For the oxidation purpose, hydrogen peroxide in the alkaline medium (H2O2/NaOH) is generally used.
Note:
It is important to note that hydroboration is a syn addition process. It means that addition of H and BH2 to alkenes takes place from the same face. It is a concerted reaction which occurs via four membered cyclic transition states.
