Question
Question: Is \(HN{O_3}\) a coordinate bond?...
Is HNO3 a coordinate bond?
Solution
The nitrogen atom is bonded to three oxygen atoms; it's bonded to at least one via one chemical bond, another via a double chemical bond, and another by a dative (coordinate) chemical bond. there's one coordinate chemical bond in aqua fortis.
Complete answer:
Ozone which isn’t attached to the hydrogen and therefore the one which isn't a double bone with nitrogen. This leaves one oxygen which has three non-bonding electron pairs of electrons. This oxygen RECEIVES the non-bonding pair that also remains on the NITROGEN atom after it's formed a covalent bond with one among the oxygens and one bond with the opposite oxygen which successively is bonded to the hydrogen.
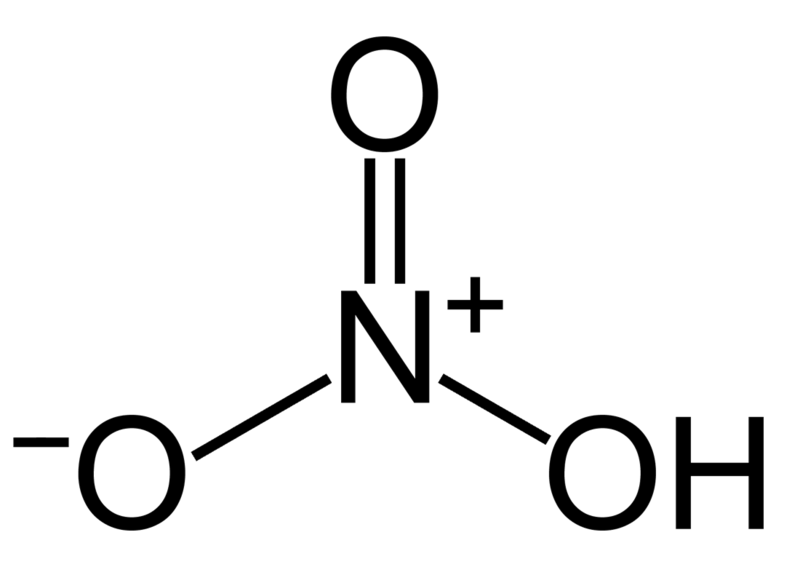
The HNO3 Lewis structure is best thought of because the NO3 with H attached to at least one of the oxygen atoms. this is often a pattern seen with many acids. For the HNO3 Lewis structure, calculate the entire number of valence electrons for the HNO3 molecule. After determining what percentage valence electrons there are in HNO3, place them around the central atom to finish the octets. make certain to use the number of obtainable valence electrons you found earlier. The HNO3 Lewis structure has 24 valence electrons.
Note:
A chemical bond is made by two atoms sharing a pair of electrons. The atoms are held
together because the electron pair is attracted by both of the nuclei. within the formation of an easy chemical bond, each atom supplies one electron to the bond - but that doesn't need to be the case.
