Question
Question: Is HCN linear, trigonal or tetrahedral?...
Is HCN linear, trigonal or tetrahedral?
Solution
This question will be solved purely on the basis of the geometry of the molecules that they acquire while stabilising their bonds and the way they exist in nature. To answer this question, we should have knowledge regarding the geometry of the molecules.
Complete Step By Step Answer:
This answer is very easy to answer, for this we should have knowledge regarding the geometry of the molecule.
So let’s solve this question, now coming to the molecule hydrogen cyanide HCN , so this is a linear molecule.
For a better understanding of the question, let’s have a look at the structure of the molecule hydrogen cyanide.
So, this is the molecule:
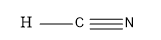
So, as we can see from the above figure that in the HCN molecule i.e. in hydrogen cyanide molecule, one electron from the hydrogen is shared with the carbon and the three electrons of the Nitrogen atom forms the triple bond with the Carbon atom and thus completing the valence of eight electrons of the carbon atom i.e. the middle atom.
So, from the above discussion , we can say that the hydrogen cyanide molecule is linear in shape and they have an angle of 180o between Cabin and hydrogen and carbon and nitrogen.
Note:
As given in questions, the trigonal planar symmetry consists of the four atoms , one at the middle of the equilateral triangle and three other at the corners of the triangle , the all there ligands or atoms are identical in shape and has a bond angle of 120o .
