Question
Question: Is \({{H}_{2}}O\) show dipole-dipole interaction?...
Is H2O show dipole-dipole interaction?
Solution
Dipole-dipole interaction is a type of interaction between the elements in a compound. Dipole-dipole interactions are only formed by those compounds which are polar, i.e., there is a difference in the atoms of the compound which creates polarity in the molecule.
Complete step-by-step answer: Dipole-dipole interaction is an intermolecular force existing among the molecules. This interaction is only formed in those compounds in which the polarity is present, i.e., those compounds which are polar. Polar molecules are those compounds in which the elements joined in the compound have different electronegativity. Like the HCl compound, the electronegativity of Hydrogen is very small while the electronegativity of chlorine is high. This creates a polarity towards the more electronegative atom.
So, a water molecule contains hydrogen and oxygen atoms, and oxygen is also an element that has high electronegativity. Therefore, there will be a difference in the electronegativities. Water is a polar molecule. The polarity in water is given below:
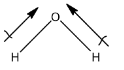
The dipole-dipole interaction is formed because the negative part of the molecule will attract the positive part of the other molecule. In water, the oxygen has a slight negative charge that will try to attract the hydrogen element of another water molecule. This interaction is water is dipole-dipole interaction.
Therefore, the given statement in the question is true, i.e., the water has dipole-dipole interaction.
Note: There are many more types of interaction like ion-dipole interaction, ion-induced dipole interaction, etc. The ion-dipole interaction is formed when the salts are dissolved in water, and ion-induced dipole interaction is formed between NO3− ion and I2.
