Question
Question: Is \( {H_2}C{O_3} \) a polar molecule?...
Is H2CO3 a polar molecule?
Solution
Molecules are the combination of atoms, one carbon atom, two hydrogen atoms, and three oxygen atoms forming a molecule known as carbonic acid. The molecular formula of carbonic acid is H2CO3 , the bonds in carbonic acid were not balanced. Thus, there is a polarity or movement of electrons.
Complete answer:
Carbonic acid is represented by a chemical formula of H2CO3 consisting of elements of carbon, hydrogen and oxygen. It can be prepared by dissolving carbon dioxide in water. The valence electrons of carbon is 4 and the valence electrons of hydrogen is 1 , as there were two hydrogen atoms, the valence electrons were 2 , oxygen has 6 valence electrons, as there were three oxygen atoms, the valence electrons were 18 valence electrons. Thus, the total valence electrons n carbonic acid were 18+2+4=24
Out of these 24 valence electrons, 12 valence electrons were involved in bonding electrons, and remaining electrons exist as lone pairs on three oxygen atoms in carbonic acid. The two C−O bonds are two dipoles which will not equal the magnitude of a dipole of C=O . Thus, there is a dipole moment in the carbonic acid molecule. The presence of a dipole moment makes the molecule a polar molecule.
The structure of carbonic acid is:
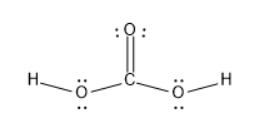
Thus, H2CO3 a polar molecule is a polar molecule.h
Note:
Carbonic acid is a weak acid as it has two hydroxyl groups with the lone pair of electrons on the oxygen atoms. It is a conjugate acid of hydrogen carbonate; it is a dibasic acid due to the presence of two −OH groups. It can also be known as chalco carbonic acid.
