Question
Question: Is cyclopropenyl cation, \( {C_3}{H_3}^ + \) aromatic?...
Is cyclopropenyl cation, C3H3+ aromatic?
Solution
Cyclopropenyl cation is a cyclopropane with a positive charge on one carbon atom formed by the loss of one hydrogen atom. The compounds that are cyclic, planar, conjugation of pi-electrons and obeying Huckel’s rule can be defined as aromatic compounds, these are stable compounds.
Complete answer:
Cycloalkenes are alkenes arranged in a cyclic manner. Alkenes are unsaturated hydrocarbons consisting of double bonded carbon atoms. When one hydrogen atom is removed from the cyclopropene, it forms a compound known as cyclopropenyl cation which is represented by a formula of C3H3+ .
Aromatic compounds are the compounds that are cyclic, planar which means all the carbon atoms have sp2 hybridization, conjugation of π electrons, and obeying Huckel’s rule. Huckel's rule states that the compound must contain (4n+2)π electrons, where n is a positive whole number.
The structure of cyclopropenyl cation (C3H3+) is
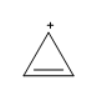
The above molecule is in cyclic arrangement, all the carbon atoms are sp2 hybridized, as the double bonded are sp2 hybridized and the carbocation is also sp2 hybridized, the double bond and the positive charge are in conjugation, and the compound has two pi-electrons which obeys Huckel’s rule as (4(0)+2)π=2π electrons. cyclopropenyl cation obeys all the conditions required to be an aromatic compound.
Thus, cyclopropenyl cation, C3H3+ is an aromatic compound.
Note:
Conjugation means the two double bonds or one double bond and charge separated by a single bond. In the above molecule, charge and double bond are separated by a single bond. Carbocation is the carbon bearing a positive charge and involves in sp2 hybridization.
