Question
Question: Is \(CC{{l}_{4}}\) covalent or ionic?...
Is CCl4 covalent or ionic?
Solution
Ionic and covalent are two types of bonds formed between the atoms in a molecule. Ionic and covalent bonds depend on the nature of the4 atoms, i.e., metal element or non-metal element. If one is metal and the other is non-metal, then it is an ionic bond, and if both of them are non-metals, then it is a covalent bond.
Complete step-by-step answer: Ionic and covalent are two types of bonds formed between the atoms in a molecule. Ionic and covalent bonds depend on the nature of the4 atoms, i.e., metal element or non-metal element. If one is metal and the other is non-metal, then it is an ionic bond, and if both of them are non-metals, then it is a covalent bond.
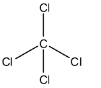
More specifically speaking, carbon tetrachloride is a nonpolar covalent compound because the electrons shared by the carbon and chlorine atoms are nearly at the center of the bond.
Therefore, the carbon tetrachloride (CCl4) is a covalent compound.
Note: Other examples of nonpolar covalent bonds are N2, O2, Cl2, etc. and some examples of the polar covalent bond are hydrochloric acid (HCl), bromine chloride (BrCl), water (H2O), etc.
