Question
Question: Is \( C{H_3}C{H_2}OC{H_2}C{H_3} \) soluble in water?...
Is CH3CH2OCH2CH3 soluble in water?
Solution
Hint : In organic chemistry, when an oxygen atom is bonded with two alkyl groups via single bonds, then the compound is characterized by the functional group known as ether. These compounds are similar to alcohols (R−O−H) in structure as there is only a difference of an alkyl group within the structure.
Complete Step By Step Answer:
Ethers are the nonpolar compounds due to the presence of alkyl groups on both sides of the central oxygen atom. If the alkyl groups bonded to the oxygen atom are bulky, then the oxygen atom is unable to participate in the hydrogen bonding and thus the boiling points are comparatively less than alcohols.
The solubility of ether depends on the number of carbon atoms present in the alkyl groups of the ether. The solubility of ether decreases with an increase in the number of carbon atoms in the alkyl chain. To be more specific, ethers containing up to three carbon atoms have the tendency to dissolve in water because of less steric hindrance and effective hydrogen bonding as shown below:
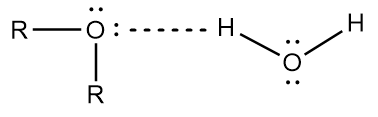
Due to its nonpolar nature, the ethers are appreciably soluble in organic solvents like alcohol, benzene, acetone, etc.
Now, as per question the given compound is CH3CH2OCH2CH3 . The chemical name of the compound is diethyl ether and because there are two carbon atoms present in the alkyl group of ether, so as per the condition for solubility of ethers given above, we can conclude that the given compound i.e., CH3CH2OCH2CH3 is soluble in water.
Note :
It is important to note that on increasing the alkyl chain of ethers, the difference in the boiling point of ether and alcohols becomes relatively smaller because on increasing the number of carbon atoms, the number of electrons increases as well and therefore, an increase in the van der Waals interactions is observed.
