Question
Question: Is \[B{F_3}\] a hypervalent compound?...
Is BF3 a hypervalent compound?
Solution
As we know that BF3 is also known as Boron trifluoride. It is named so since it has three fluorine atoms. This pungent colorless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds. The geometry of a molecule of BF3 is trigonal planar.
Complete answer:
We have to know that the BF3 is not a hypervalent molecule. If we break down BF3 and look at its atomic structure it has 6 electrons in its outermost shell. Thus, the octet of the central atom is not complete meaning the valence shell has less than 8 e−electrons. Now if we recall the definition of a hypervalent molecule it is described as a molecule containing one or more main group elements with more than eight electrons in its valence shells. A hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. For hypervalent compounds in which the ligands are more electronegative than the central, hypervalent atom, resonance structures can be drawn with no more than four covalent electron pair bonds and completed with ionic bonds to obey the octet rule. The covalent bond in BF3 tells us that electrons are shared, rather than lost by boron and gained by fluorine.
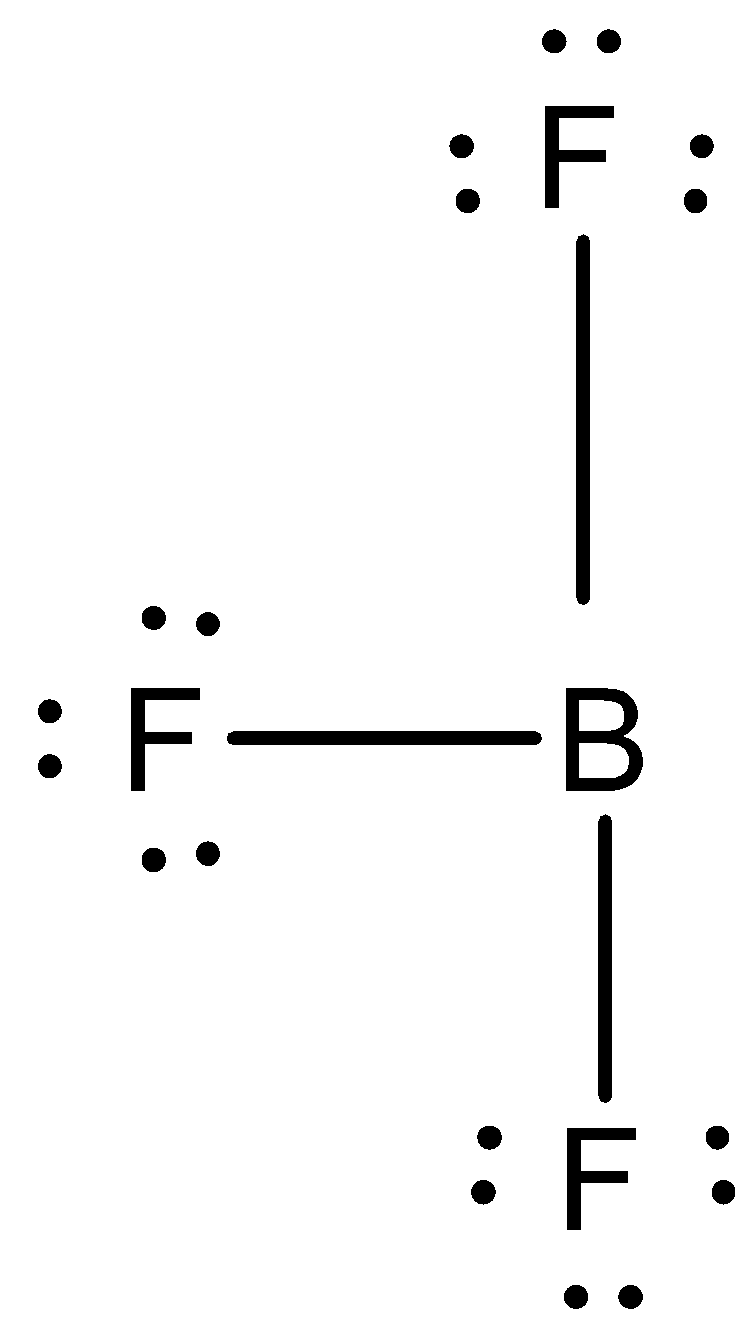
Note:
We need to know that the boron trifluoride is most importantly used as a reagent in organic synthesis, typically as a Lewis acid. Examples include: initiates polymerisation reactions of unsaturated compounds, such as polyethers as a catalyst in some isomerization, acylation, alkylation and other reactions.
