Question
Question: Is \({B_2}{H_6}\) an electrophile?...
Is B2H6 an electrophile?
Solution
Electrophile is an electron loving species. Electrophile is an electron deficient molecule or ion and can accept the pair of electrons easily from a nucleophile (electron rich molecule or ion) to make a covalent bond. Hence to find out whether B2H6 is an electrophile or not, we have to see its electronic configuration.
Complete answer:
Now, we know that an electrophile is a molecule or ion which is electron deficient in nature and hence, we can say that, these species can easily accept lone pairs of electrons from a nucleophile and form a covalent bond.
Atoms with less than octet rule electrons are considered as electron deficient in nature.
Now, if we look at the diborane molecule, B2H6 , it requires 14 electrons to follow the octet rule, but only 12 electrons are present in this molecule. Hence, we can say that this molecule is an electron deficient molecule.
Let, us see the structure of diborane:
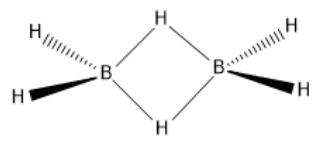
Each bond formation ideally requires two electrons. But, in the above diborane structure, we can see that both the B-H-B bonds are 3−centre,2−electron bond, hence, it is an electron deficient compound and can easily accept electrons. This type of bonding is also known as banana bonding.
Hence, we can say that diborane is a strong electrophile.
Note:
The compounds which have less number of electrons than eighteen are called electron deficient compounds or hypervalent compounds in nature whereas the compounds which have more number of electrons than eighteen are called hypervalent compounds or electron rich compounds in nature.
