Question
Question: Iron carbonyl, \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is: A. Trinuclear B. Mononuclear ...
Iron carbonyl, Fe(CO)5 is:
A. Trinuclear
B. Mononuclear
C. Tetranuclear
D. Dinuclear
Solution
Hint: Here, we will proceed by defining the nuclearity of the coordinate compounds. Then draw the structural formula of Iron carbonyl Fe(CO)5 to find the nuclearity of the given coordinate compound.
Complete answer:
Definition of Nuclearity: The nuclearity of a single coordination entity indicates the number of central atoms joined by bridging ligands or metal-metal bonds.
The simplest nuclearity is mononuclear, followed by dinuclear, trinuclear, tetranuclear, pentanuclear…………………………., polynuclear.
Iron pentacarbonyl, also known as Iron carbonyl, is the compound with compound with formula Fe(CO)5.
The structural formula of Iron carbonyl Fe(CO)5 is:
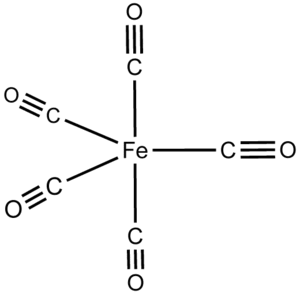
From the structural form of Iron carbonyl Fe(CO)5, one Fe atom is surrounded by 5 CO ligands.
So, Iron carbonyl Fe(CO)5 is mononuclear.
Note: Under standard conditions Fe(CO)5 is a free-flowing, straw-coloured liquid with a pungent smell. Mononuclear complexes are the simplest types of coordination compounds that contain a single metal atom or ion surrounded by monodentate ligands.
