Question
Question: Iron carbonyl, \(Fe{{\left( CO \right)}_{5}}\) is ______. (A) trinuclear (B) tetranuclear (C) ...
Iron carbonyl, Fe(CO)5 is ______.
(A) trinuclear
(B) tetranuclear
(C) dinuclear
(D) mononuclear
Solution
For this particular illustration, we need to have a basic knowledge about what we mean by the terms as mentioned above. Based on the number of atoms present in the centre, complexes are classified as mononuclear, dinuclear, etc.
- Considering one, a mononuclear molecule means it has a single metal atom surrounded by other ligands. One single nucleus of the metal atom is responsible for the name as mononuclear and so on.
Complete Solution :
Let us see about the molecule, Fe(CO)5
Iron pentacarbonyl-
Fe(CO)5 (also known as iron carbonyl) is a molecule in which one Fe atom is being surrounded by the five CO ligands. This can be shown by the diagram below:
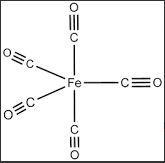
Another proof to show the nature given molecule is given by EAN rule:
EAN (Effective Atomic Number) rule-
As stated by Sidgwick, the EAN rule gives the total number of electrons involved in the complex formation; and the stable complex is shown by the number of electrons reaching the nearest stable noble gas values.
In short, a metal atom tends to surround itself by sufficient number of ligands which would result into an effective atomic number which would be equal to the atomic number of nearest noble gas and thus, the rule will be satisfied.
EAN = Z – O.N. + 2 (C.N.)
where,
Z = atomic number
O. N. = oxidation number
C. N. = coordination number
For Fe(CO)5-
EAN = 26 – 0 + 2 (5) = 36
Hence, we can say that Fe(CO)5 is mononuclear.
So, the correct answer is “Option D”.
Note: Fe(CO)5 is toxic in nature and inhalation can cause lung irritation. So, proper care must be taken in the environment where the compound is present.
