Question
Question: Ionic hydrides are formed by: (A) Transition metals (B) Elements of very high electropositivity ...
Ionic hydrides are formed by:
(A) Transition metals
(B) Elements of very high electropositivity
(C) Elements of very low electropositivity
(D) Metalloids
Solution
Hint: Stoichiometric compounds of hydrogen are formed with those elements of the periodic table whose valence shells have one or two electrons and do not form coordination complexes too. (stoichiometric means relative moles of different reactants react with each other in a balanced chemical equation).
Complete step by step answer:
The elements which form ionic hydrides are the elements of the s-block of the periodic table which are highly electropositive in nature.
Some of the properties of the ionic hydrides are – crystalline structure, non-volatile, non-conductor in solid state, white, colourless, high melting and boiling point and decomposed by water, alcohol very easily.
These are formed by the alkali and alkaline earth metals (Gr-I and Gr-II) except Beand Mg.
2Na+ H2 573K 2NaH
2K+ H2 673K 2KH
Ca+ H2 1073K CaH2
Sr+ H2 1173K SrH2
So, the correct option is B.
Additional information:
Transition metals form covalent hydrides which are also known as interstitial hydrides.
There are three types of hydrides-
- Ionic hydrides (salt-like hydrides)
- Covalent hydrides (molecular hydrides)
- Metallic hydrides (non-stoichiometric hydrides)
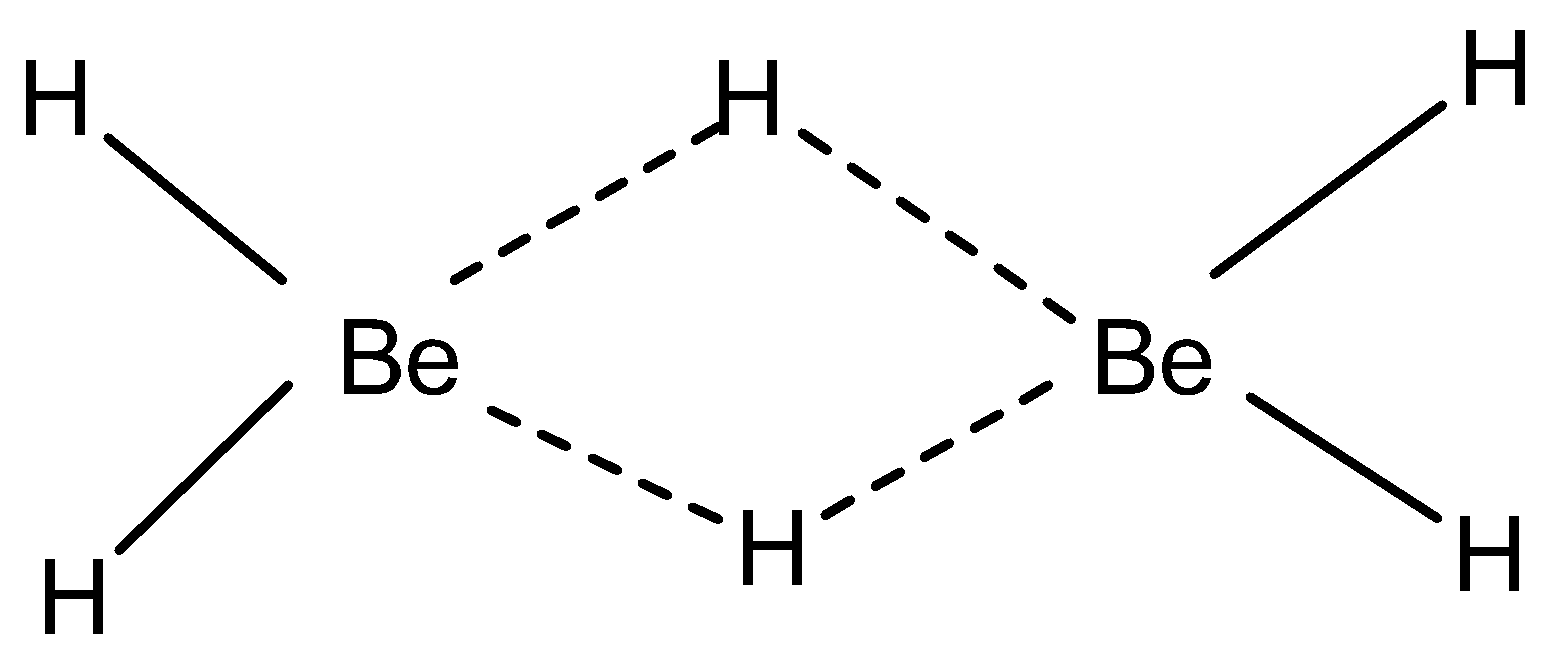
Note: LiH decomposes at 400−500∘C (exception). Mg and Be form polymeric hydrides because of the small electronegativity difference. Because of their small size, these metals are covalent in nature and form bridging or polymeric hydrides due to covalent bonding.
Be occurs as (BeH2)n also known as diborane. MgH2 occurs as 3-D structure also known as rutile structure.