Question
Question: Iodoform reaction is answered by all except: [A] \(C{{H}_{3}}CHO\) [B] \(C{{H}_{3}}C{{H}_{2}}C{{...
Iodoform reaction is answered by all except:
[A] CH3CHO
[B] CH3CH2CH2OH
[C] CH3CHOHCH2COOH
[D] CH3CH2OH
Solution
Iodoform is generally shown by ketones, aldehyde and also alcohol. The only aldehyde which shows positive results in iodoform test is acetaldehyde and the only alcohol which shows positive results is ethanol.
Complete step by step answer:
Iodoform test is used in laboratories for the detection of aldehydes and ketones containing an alpha methyl group.
Compounds containing CH3CO or CH3CH(OH) groups show positive results in iodoform test.
When iodine and sodium hydroxide are added to a compound containing methyl ketone or a secondary alcohol having a methyl group in alpha position, a yellow iodine precipitate is obtained due to formation of triiodomethane. The reaction is:
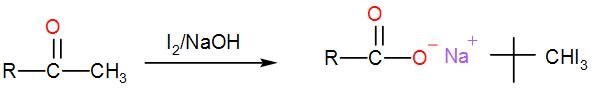
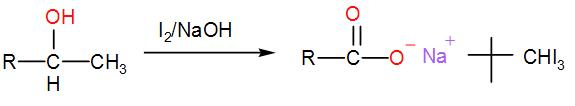
Now, let us check which of the given options show iodoform test;
In option [D], we have CH3CH2OH
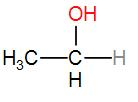
It is ethanol and it contains a CH3CH(OH) group. Therefore iodoform will be answered by it.
In option [C], we have CH3CHOHCH2COOH
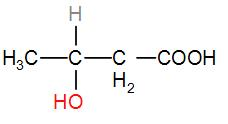
It has a CH3CH(OH) group, therefore the iodoform test will be answered by it.
In option [B], we have CH3CH2CH2OH
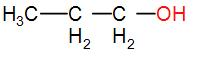
It does not have either CH3CO nor CH3CH(OH) group. Therefore, it will not show the iodoform test.
Lastly, in option [A] we have, CH3CHO
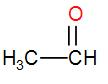
It has a CH3CO group, therefore it will show iodoform test too.
Therefore, the only compound which will not show positive result in iodoform test is CH3CH2CH2OH.
Therefore, the correct answer is option [B] CH3CH2CH2OH
Note:
Iodoform test is used to detect ethanol or ethanal (acetaldehyde) as they are the only alcohol and aldehyde respectively which show the iodoform test. Solution to such questions can be easily found just by drawing the structure but we have to remember that this test is shown only by the compounds containing CH3CO or CH3CH(OH) group.
