Question
Question: Intermolecular Hydrogen bond is present in which of the following pair of molecules? Option- A.\...
Intermolecular Hydrogen bond is present in which of the following pair of molecules?
Option-
A.SiH4 and SiF4
B.CH3COCH3 and CHCl3
C.HCOOH and CH3COOH
D.CH3OCH3 and H2O2
Solution
Hydrogen bond is formed between the hydrogen atom and any highly electronegative element. Generally, fluorine, oxygen and nitrogen atoms frequently form hydrogen bonding. Intermolecular and intramolecular hydrogen bonding are two different types of hydrogen bonding.
Complete answer:
Intermolecular hydrogen bond is usually formed between the two different molecules. Hydrogen atoms from one molecule interact with highly electronegative elements like fluorine, oxygen or nitrogen atoms of different molecules. Therefore, at least two molecules are required to form intermolecular hydrogen bonding.
In the case of SiH4and SiF4 molecules, silicone tetrahydride and silicon tetrafluoride are hydrides of group (IV). All the hydrides of this group are non-polar in nature and therefore they are not capable of forming hydrogen bonds. If under certain circumstances they form the hydrogen bond it is very weak in nature.
In the case of CH3COCH3 and CHCl3, acetone is polar molecule because two methyl group are electron donor and impart partial positive charge on oxygen atom, which further forms hydrogen bonding but due to large size atom of chlorine atom in chloroform molecule it is not able to interact with oxygen atom of acetone to form intermolecular hydrogen bonding. Therefore, due to the large size of chlorine atoms it does not participate in intermolecular hydrogen bonding.
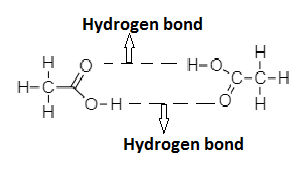
In the case of HCOOH and CH3COOH, the carbonyl oxygen atom of acid interacts with the hydrogen atom of the hydroxyl group of other molecules. This will result in formation of two hydrogen bonds between HCOOH and CH3COOH. Due to formation of intermolecular hydrogen bonding between acid molecules, it is capable of existing as a dimer even in the gaseous state.
In the case of CH3OCH3 and H2O2 molecules, dimethyl ether is non polar molecule and do not have any dipole moment and it does not have sufficient electronegativity to form hydrogen bond with the oxygen atom present in hydrogen peroxide molecule. Therefore, they do not participate in the formation of intermolecular hydrogen bonding.
⇒ HCOOH and CH3COOH molecules form intermolecular hydrogen bonding.
Therefore, option (C) is the correct option.
Note:
Intramolecular hydrogen bonding is usually formed within the same molecule between the hydrogen atom and any highly electronegative element. Intramolecular hydrogen bonding is observed in the molecule of ortho nitrophenol and ortho nitrobenzoic acid.
