Question
Question: Inorganic graphite is: (A). \[{\left( {BN} \right)_n}\] (B) \(B{F_4}\) (C) \({B_2}{H_6}\) ...
Inorganic graphite is:
(A). (BN)n
(B) BF4
(C) B2H6
(D) B2N2H6
Solution
Inorganic graphite is an inorganic compound whose structure is like graphite with alternating atoms replacing the carbon atoms in hexagonal structure.
Complete step by step answer:
Boron nitride is sometimes referred to as ‘inorganic graphite’ because its structure is like graphite with alternating boron and nitrogen atoms replacing the carbon atoms in the hexagonal structure.
BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms.
The structure of BN is
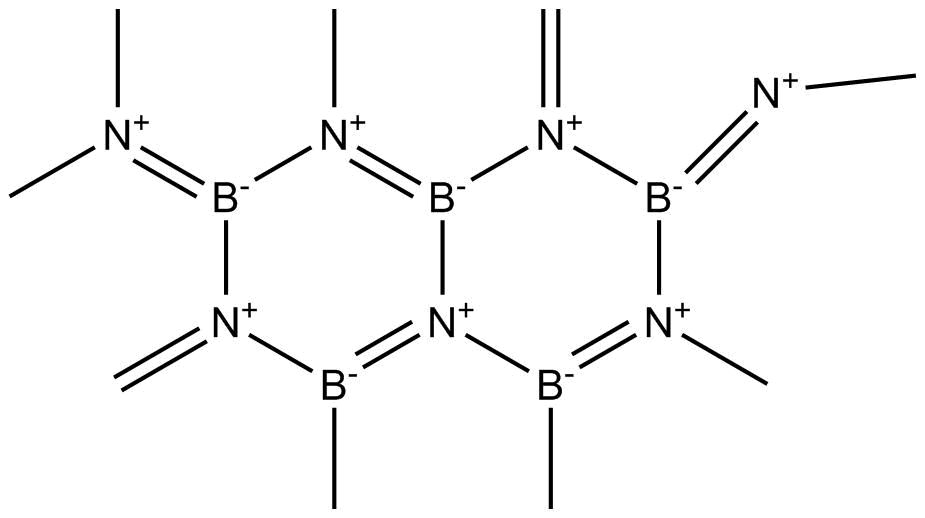
Figure-The structure of Boron Nitride
Additional information:
Boron nitride is produced synthetically. Hexagonal boron nitride is obtained by the reacting trioxide (B2O3) or boric acid (H3BO3) with ammonia (NH)3 or (NH2CONH2) in a nitrogen atmosphere.
B2O3+2NH3→2BN+3H2O (T=900∘c)
B(OH)3+NH3→BN+3H2O (T=900∘c)
B2O3+CO(NH2)2→2BN+CO2+2H2O (T>1000∘c)
B2O3+3CaB6+ION2→2OBN+3CaO (T>1500∘c)
Similar to graphite, various molecules such as NH3 or alkali metals can be intercalated into hexagonal boron nitride, that is inserted between its layers. Both experiment and theory suggest the intercalation is much more difficult for BN than for graphite.
Note:
BN is the most widely used polymorph. It is a good lubricant at both low and high temperature. BN is used as an lubricant when electrical conductivity or chemical reactivity of graphite would be problematic.
