Question
Question: Incorrect statement about pyrophosphorous acid \({{H}_{4}}{{P}_{2}}{{O}_{5}}\) is: (A) It contains...
Incorrect statement about pyrophosphorous acid H4P2O5 is:
(A) It contains P in +5 oxidation state.
(B) It is dibasic.
(C) It is strongly reduced in nature.
(D) It contains one P−O−P bond.
Solution
Pyrophosphorous acid H4P2O5 is an oxoacid of phosphorus. All the oxoacids of phosphorus have at least one P=O and one P−OH bond in their structure.
Complete step by step answer:
In oxoacids, phosphorus have four groups or atoms arranged in a tetrahedron. Structure of pyrophosphorous acid H4P2O5 is given below:
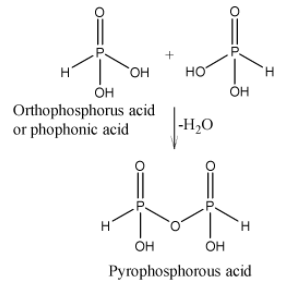
Some of the important characteristics of pyrophosphorous acid H4P2O5 are discussed below:
It is clear from the structure given above that H4P2O5 contains two P=O, two P−H, two P−OH and one P−O−P bond.
Oxidation state of P in H4P2O5 can be calculated as follows:
Let the oxidation state of P be ‘x’. Oxidation state of H is +1 and of O is taken as -2.
4×1+2x+5×(−2)=0
4+2x−10=0
2x−6=0
x=3
Therefore, P is present in +3 oxidation state.
The O−H bonds present in H4P2O5 are ionizable and give H+ ions. Basicity of oxoacids depends on the number of H+ ions. Thus, the basicity of H4P2O5 is 2 i.e. it is a dibasic acid.
Greater the number of P−H bonds, stronger is the reducing agent. H4P2O5 contains two P−H bonds thus, it is strongly reduced in nature.
Based on the above discussed points, it is clear that the oxidation state of phosphorus in pyrophosphorous acid is +3 and not +5.
So, the correct answer to the question is option (A).
Note: Carefully calculate the oxidation state and the number of different bonds present. Do not confuse pyrophosphorous acid with any other oxoacid of phosphorus.
