Question
Question: In Wurtz-Fittig reaction, the phenol is reduced to benzene A.True B.False...
In Wurtz-Fittig reaction, the phenol is reduced to benzene
A.True
B.False
Solution
To answer this question, you should recall the concept of Wurtz-Fittig Reaction. The Wurtz-Fittig reaction mechanism is explained either via the organo-alkali mechanism or the radical mechanism. It involves addition of two alkyl halides in the presence of sodium.
Complete step by step answer:
Wurtz Fittig reaction involves SN2 mechanism. This reaction proceeds through a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of180o to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
The reaction mechanism of the reaction of phenol undergoing Wurtz-Fittig reaction can be shown by:
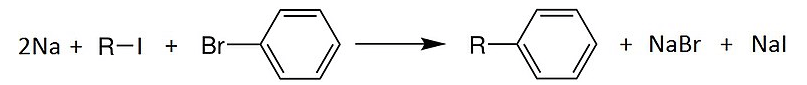
Hence, the statement given in the question is false.
Thus, the correct option is option B.
Additional information:
Important points regarding SN2 reactions:
SN2 reactions are bimolecular reactions in which there are simultaneous bond-making and bond-breaking steps.
SN2 reactions do not proceed via an intermediate.
SN2 reactions result in inverted stereochemistry at the reaction centre.
Steric effects are particularly important in SN2reactions.
Unhindered back of the substrate makes the formation of carbon-nucleophile bond easy. Therefore, methyl and primary substrates undergo nucleophilic substitution easily.
Strong anionic nucleophiles speed up the rate of the reaction. More negative charge more the nucleophilicity and a strong nucleophile can easily form the carbon-nucleophile bond.
Note:
Polar aprotic solvents do not hinder the nucleophile, but polar solvents form hydrogen bonds with the nucleophile. A good solvent for this reaction is acetone.
Stability of the anion of the leaving group and the weak bond strength of the leaving groups bond with carbon help increase the rate.
