Question
Question: In Williamson's synthesis, ethoxy ethane is prepared by, A.Heating sodium ethoxide with ethyl bro...
In Williamson's synthesis, ethoxy ethane is prepared by,
A.Heating sodium ethoxide with ethyl bromide
B.Passing ethanol over heated alumina
C.Treating ethyl alcohol with excess of conc. H2SO4 at 430 – 440 K
D.Heating ethanol with dry Ag2O
Solution
We know that Williamson's synthesis is one of the best methods for the preparation of both simple and mixed ethers. It’s a coupling reaction. The reaction was developed by Alexander William Williamson.
Complete answer:
We know that Williamson's synthesis is the reaction of alkyl halides with sodium alkoxide or sodium phenoxide to form ethers.
Let’s now see the chemical reaction in Williamson’s synthesis .
For example, For example, R−X+R′−ONa△R−O−R′+NaX
The Alkyl halide reacts with the sodium alkoxide in presence of heat to produce Ether and sodium halide as a byproduct.
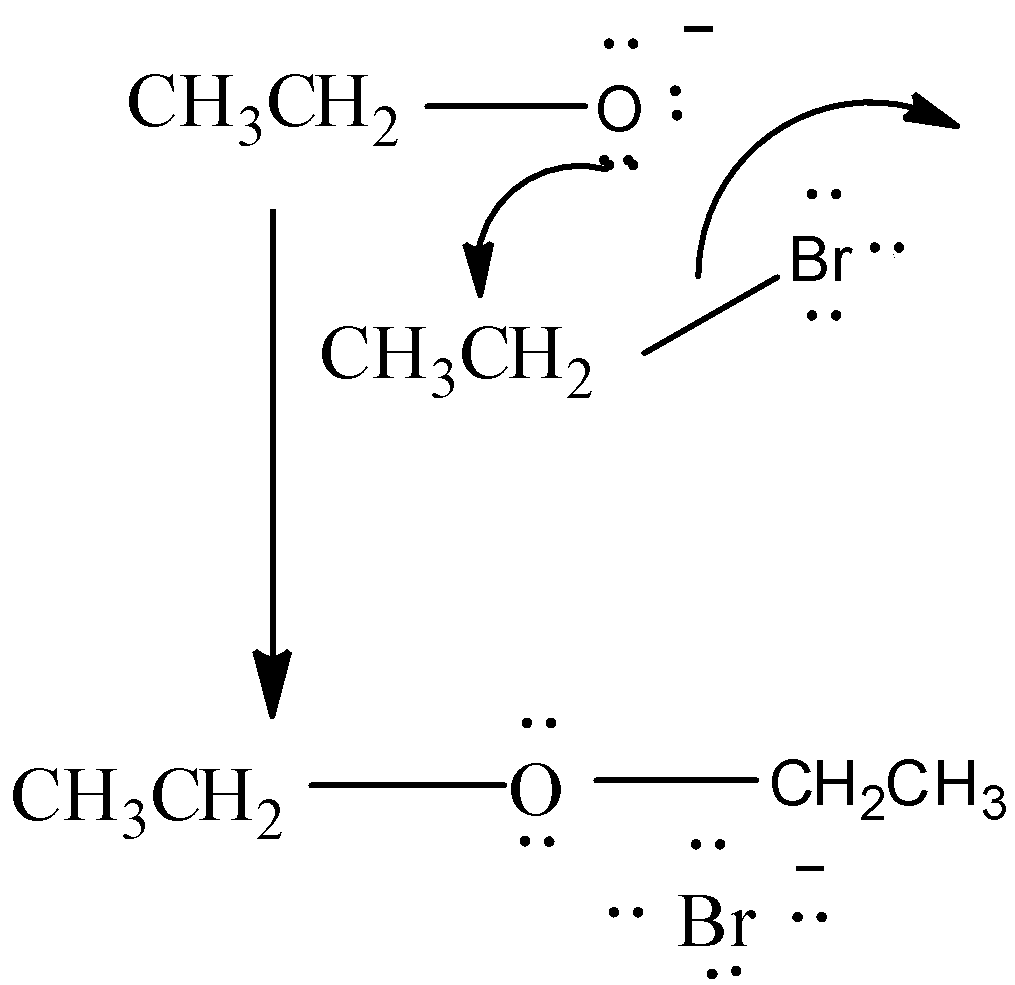
CH3CH2Br+CH3CH2ONa△CH3CH2−O−CH2CH3+NaBr
At first, we get Sodium ethoxide ( CH3CH2O−Na+) by passing metallic Na through Ethanol (CH3CH2OH) . Then, Ethyl Bromide reacts with Sodium ethoxide to form Ethoxy ethane (IUPAC Name: Diethyl ether) viaSN2 reaction.
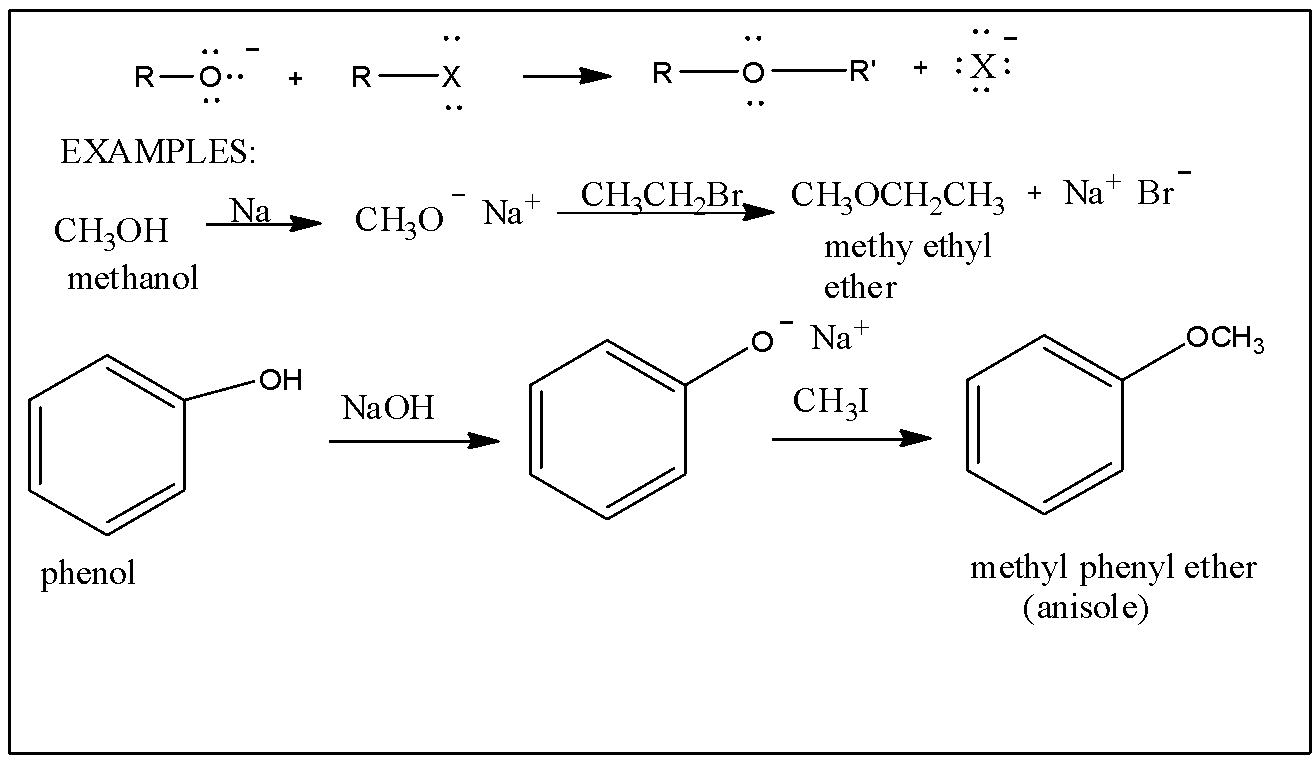
The correct option is A. Heating Sodium ethoxide with Ethyl bromide.
Note: Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical reaction. If higher degree of alkyl halide is used i.e. Secondary alkyl halide and Tertiary alkyl halide then, the reaction will go through E2 type of reaction instead of SN2 Reaction and result in the formation of a Alkene in the product section.
