Question
Question: In which pairs, the first ion is more stable than the second?...
In which pairs, the first ion is more stable than the second?
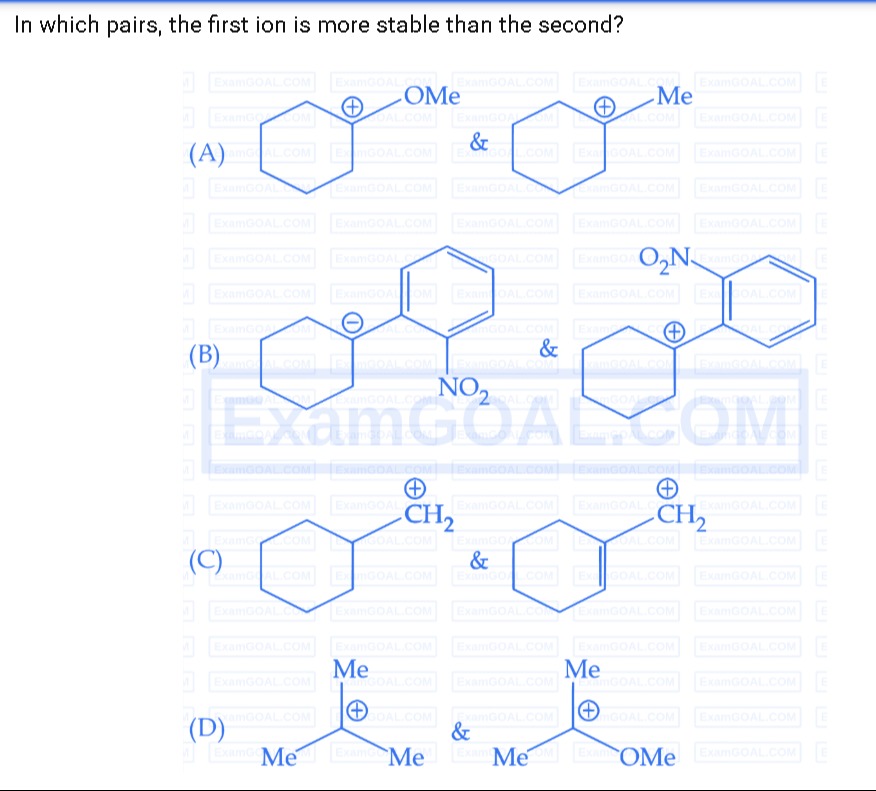
A
Pair A: Cyclohexyl carbocation with OMe substituent vs cyclohexyl carbocation with Me substituent
B
Pair B: Cyclohexyl carbanion with p-NO₂-phenyl substituent vs cyclohexyl carbocation with p-NO₂-phenyl substituent
C
Pair C: Two primary cyclohexylmethyl carbocations in different conformations
D
Pair D: t-Butyl carbocation vs t-Butyl carbocation bearing an OMe group
Answer
Pair B: Cyclohexyl carbanion with p-NO₂-phenyl substituent vs cyclohexyl carbocation with p-NO₂-phenyl substituent
Explanation
Solution
Key concept: Nitro group (–NO₂) is a strong electron-withdrawing group by both inductive and resonance effects.
- In the first ion of Pair B, the nitro group stabilizes the carbanion by delocalizing and withdrawing negative charge (–I and –R effects).
- In the second ion, the nitro group destabilizes the carbocation (positive charge), since electron withdrawal from a positive center exacerbates instability.
Hence, the first ion is significantly more stable than the second in Pair B.
