Question
Question: In which pair of species, the octet rule is not obeyed? A. \(P{F_3}\) and \(POC{l_3}\) B. \(BC{l...
In which pair of species, the octet rule is not obeyed?
A. PF3 and POCl3
B. BCl3 and PCl5
C. CF4 and NF3
D. NH3 and NCl3
Solution
We can find the species that does not follow the octet rule by calculating the total number of electrons in the species. The total number of electrons in species is calculated by adding the number of bonded electrons and the number of electrons in the lone pairs.
Complete step by step answer:
We can say a stable arrangement is attained when the atom is surrounded by eight electrons. This octet could be made up of its own electrons and some electrons that are shared. Therefore, an atom continues to form bonds until an octet of electrons is made
Let us now calculate the total number of electrons present in all compounds by adding the electrons in bond pairs and lone pairs.
In the pair (a),
PF3 contains six electrons in its bond pair and there is one lone pair (two electrons) present in phosphorus, so a total of eight electrons are obtained. PF3 satisfies the octet rule. We can draw the structure of this compound as below,
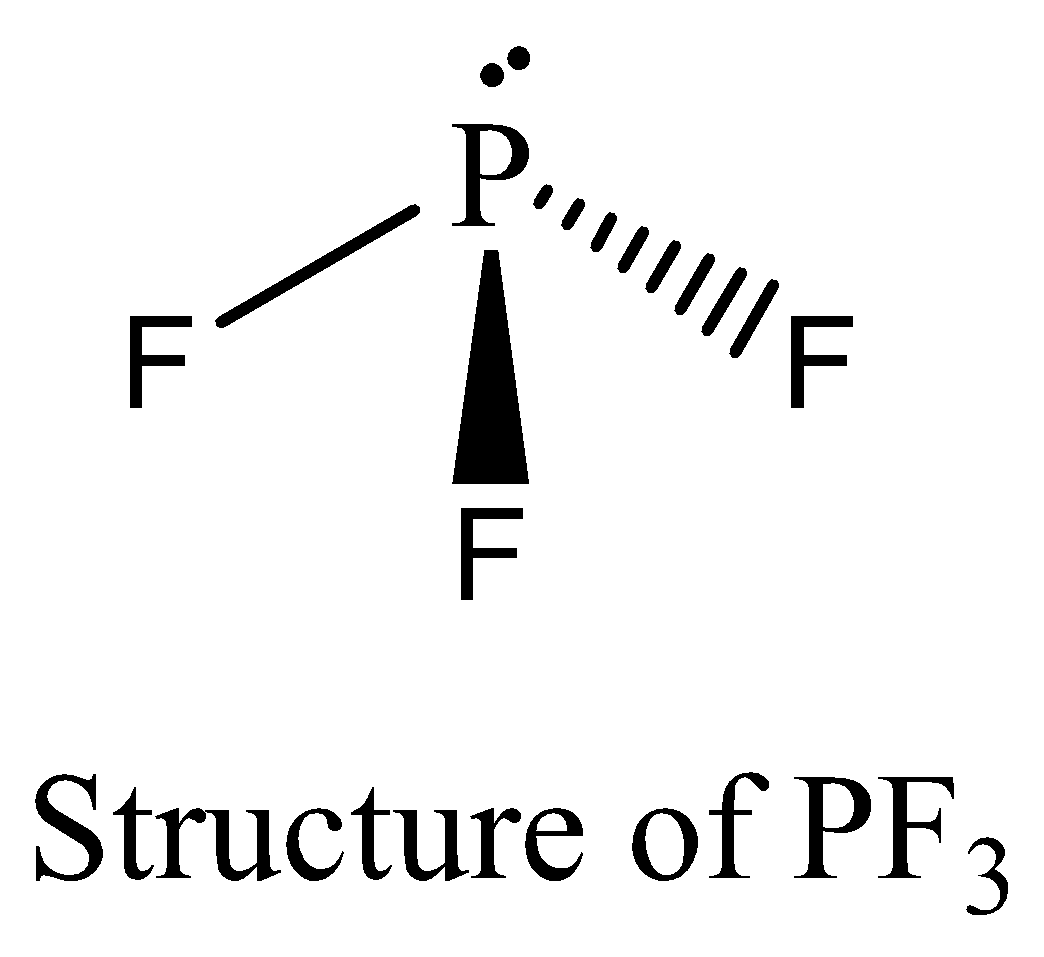
POCl3 contains 8 bonding electrons and no lone pair of electrons are present. The octet rule is satisfied by POCl3. We can draw the structure of this compound as below,
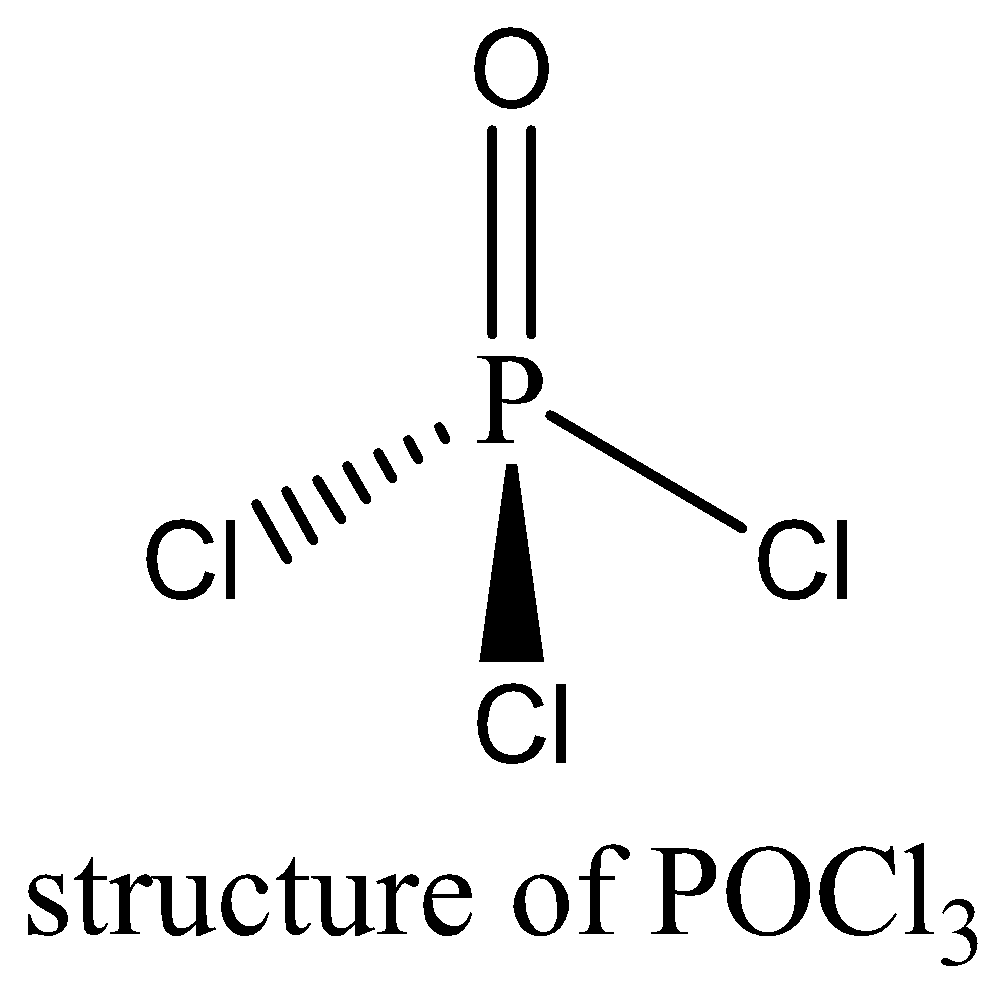
Therefore, the option (A) is incorrect.
In the pair (b),
BCl3 contains six electrons in its bond pair and there is no one lone pair present in boron, so a total of eight electrons are not obtained. The octet rule is not satisfied by BCl3. We can draw the structure of this compound as below,
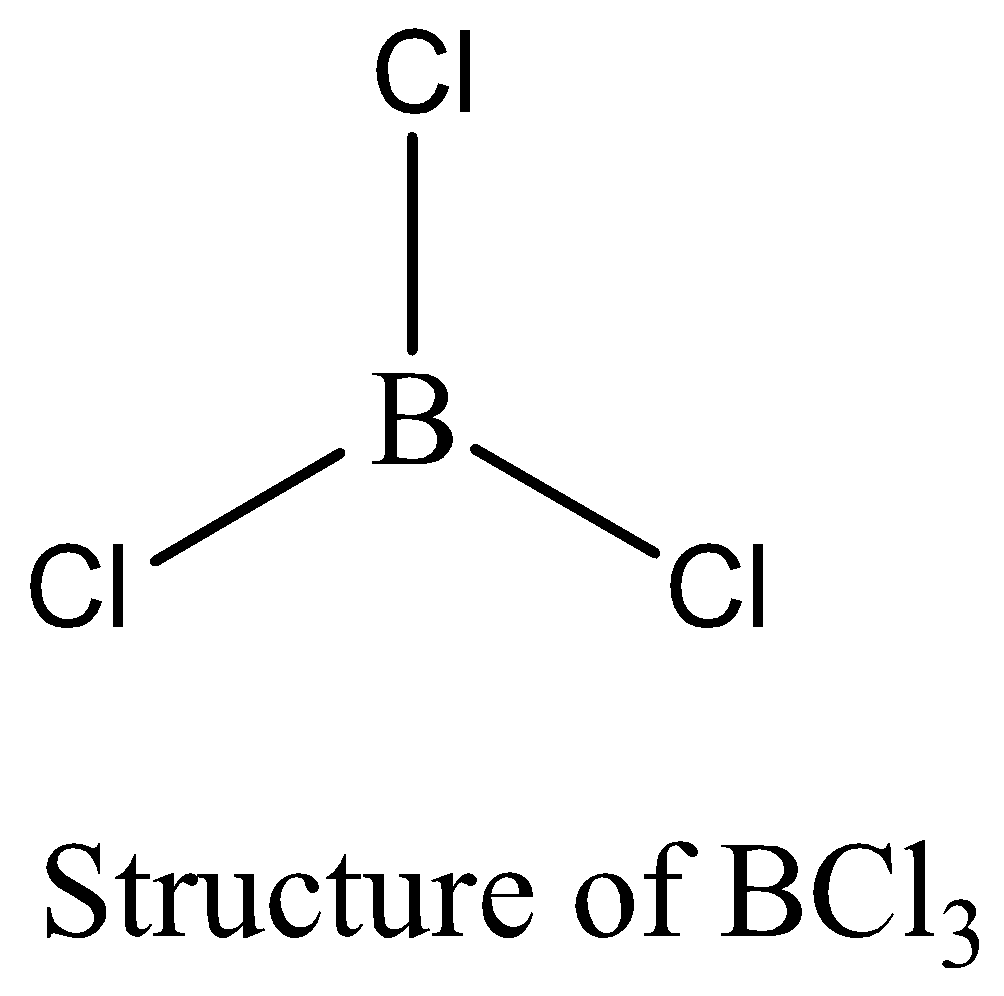
PCl5 contains 10 numbers of bonding electrons and no lone pair of electrons are present. The octet rule is not satisfied by PCl5. Therefore, the option (B) is correct. We can draw the structure of this compound as below,
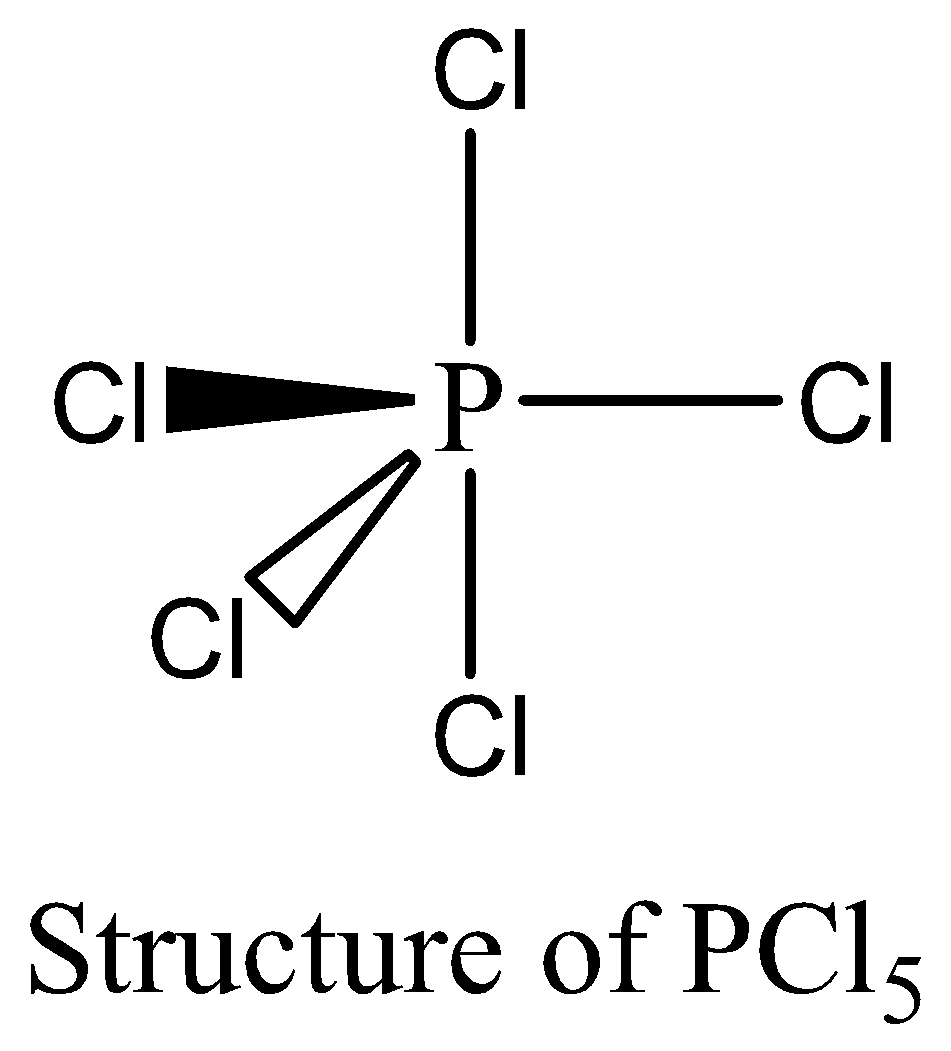
In the pair (c),
CF4 contains eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. The octet rule is satisfied by CF4. We can draw the structure of this compound as below,
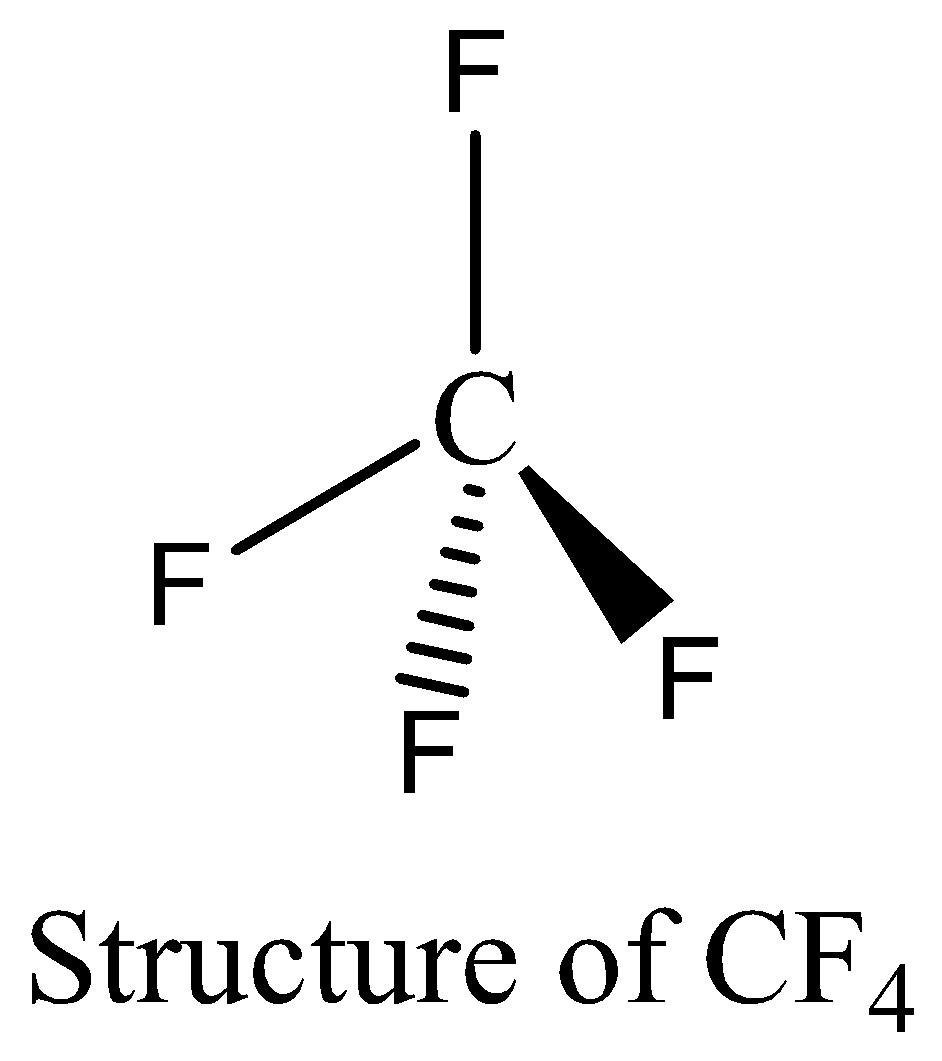
NF3 contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by NF3.
We can draw the structure of this compound as below,
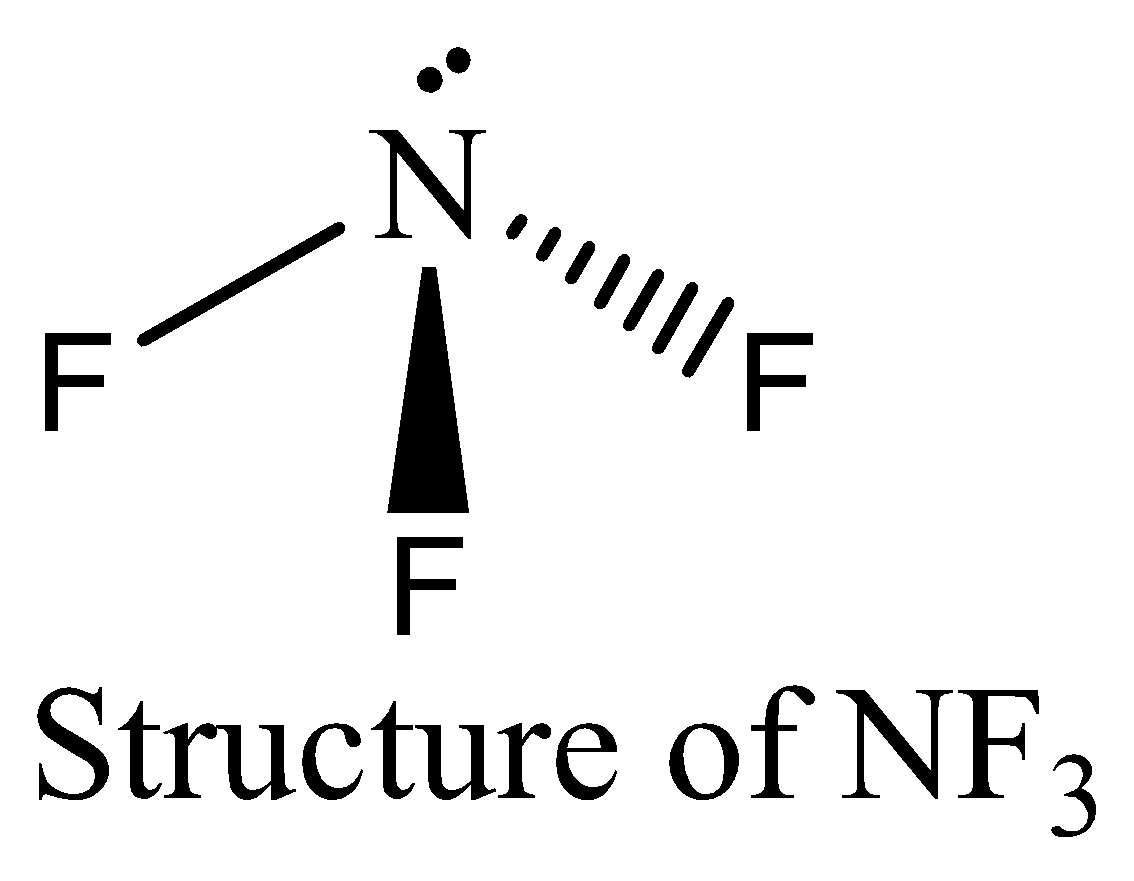
Thus option (C) is incorrect.
In the pair (d),
NH3 contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by NH3. We can draw the structure of this compound as below,
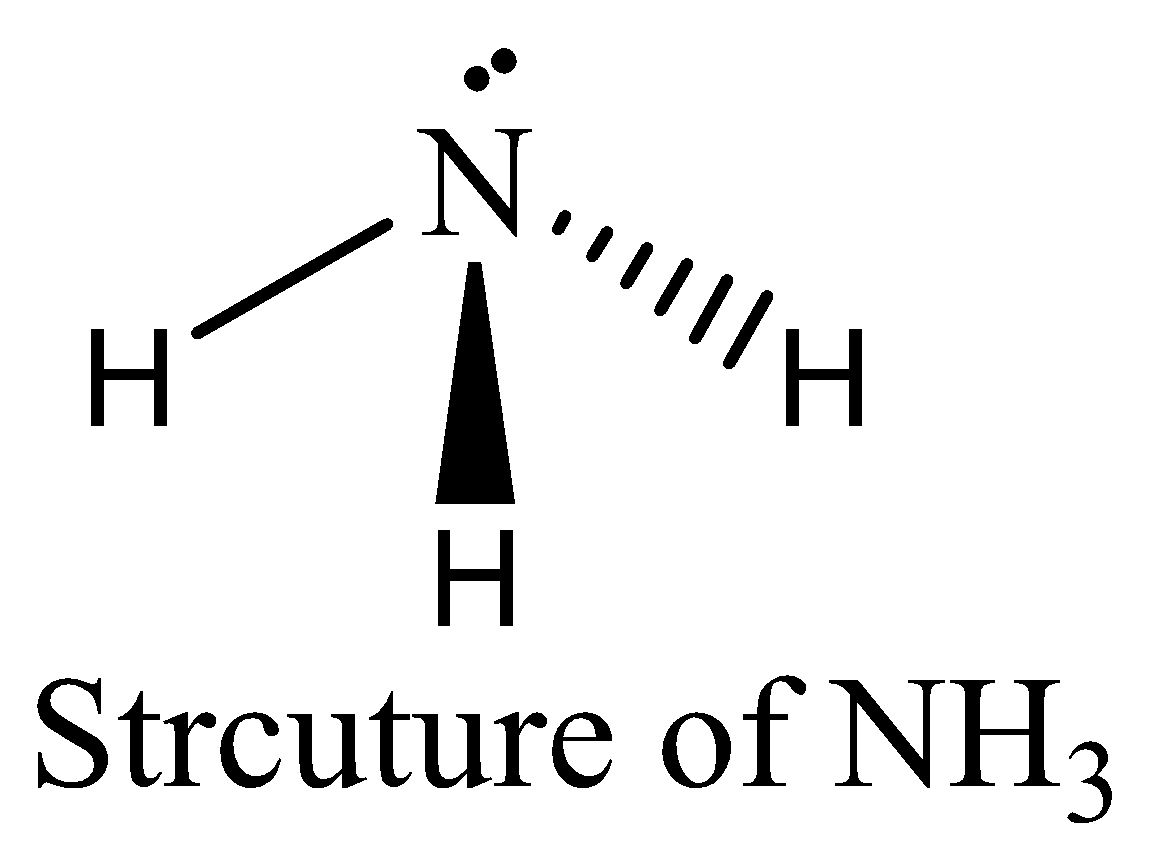
NCl3 contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by NCl3. We can draw the structure of this compound as below,
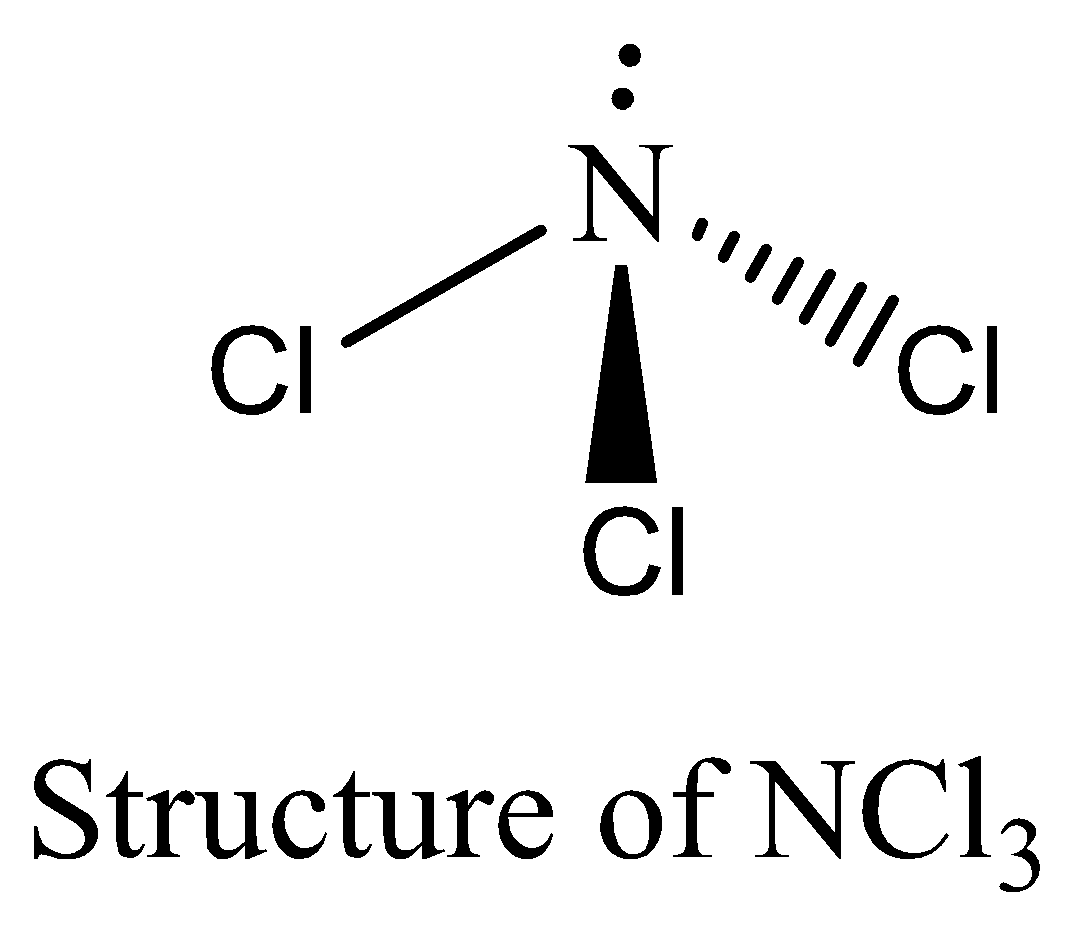
Therefore, the option (D) is incorrect.
The pair of species, which does not follow the octet rule is BCl3 and PCl5.
Therefore, the correct option is B. .
Note: We must remember that in SF6 the atom that violates octet rule is sulfur. The central sulfur atom forms six covalent bonds to six fluorine atoms, therefore it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule SF6 violates the octet rule. In BH3 the atom that deviates octet rule is boron. There are only six outermost electrons in BH3 around the central atom boron. The atom of boron has incomplete octet and hence, the molecule BH3 violates the octet rule.
