Question
Question: In which of the following sulfur is present in the +5 oxidation state? (a)- Dithionic acid (b)- ...
In which of the following sulfur is present in the +5 oxidation state?
(a)- Dithionic acid
(b)- Sulfurous acid
(c)- Sulfuric acid
(d)- Disulfuric acid
Solution
All the options are the oxoacids of sulfur. The oxidation state of sulfur can be calculated by equating the sum of all oxidation states of all atoms in the molecule to the overall charge of the molecule.
Complete step by step answer:
Let us see all the options one by one:
(a)- Dithionic acid
The formula of Dithionic acid is H2S2O6. The structure of Dithionic acid is given below:
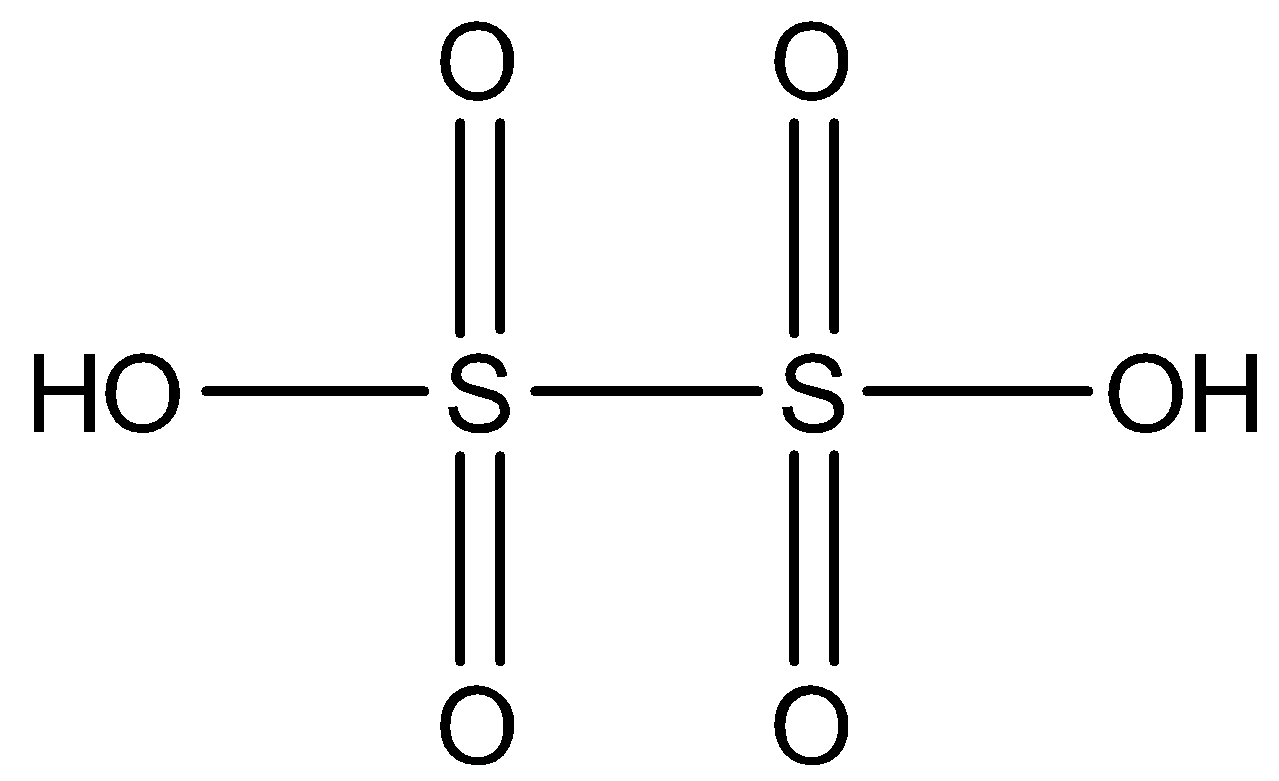
So, to find the oxidation state of sulfur in Dithionic acid,
Let us assume the oxidation state of sulfur is x and the overall charge of the molecule is zero.
Therefore, 2(+1) + 2(x) + 6(-2) = 0
2+2x−12=0
x=+5
So, the oxidation state of sulfur in Dithionic acid is +5.
(b)- Sulfurous acid.
The formula of Sulfurous acid is H2SO3. The structure of Sulfurous acid is given below:
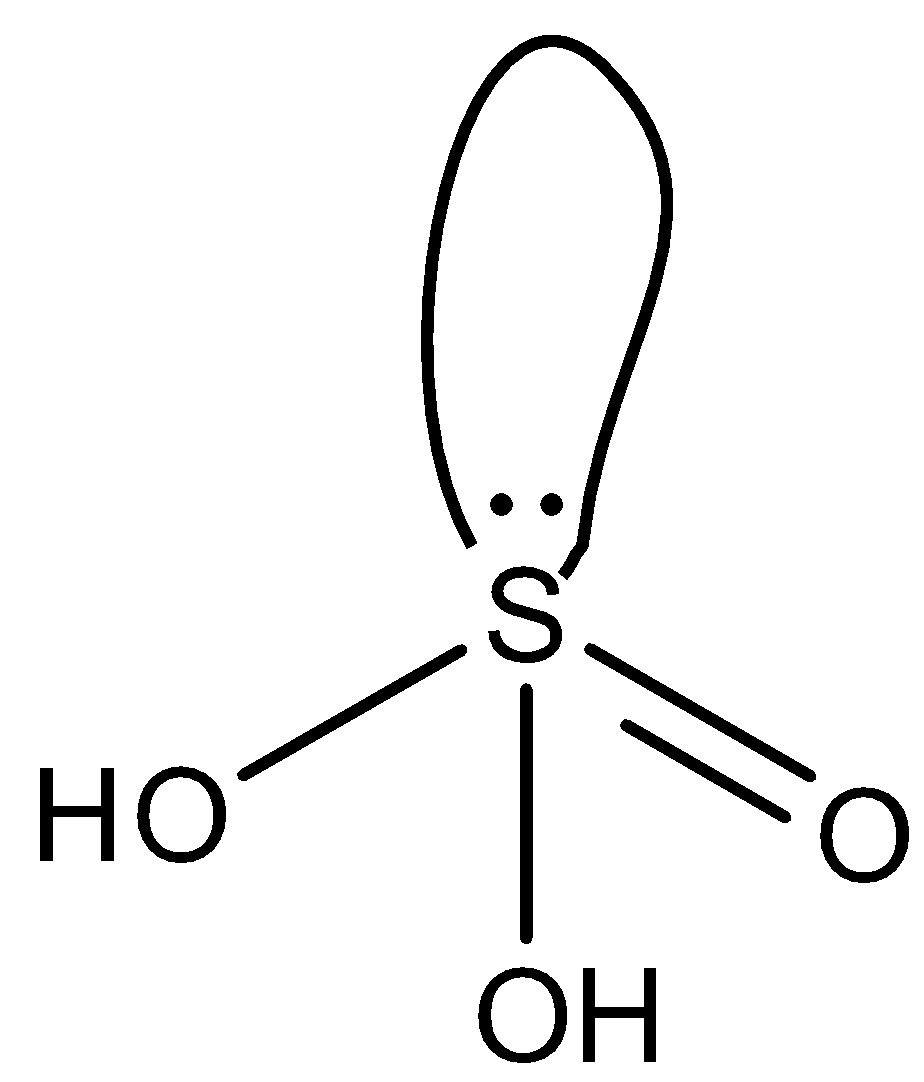
So, to find the oxidation state of sulfur in Sulfurous acid,
Let us assume the oxidation state of sulfur is x and the overall charge of the molecule is zero.
Therefore, 2(+1) + (x) + 3(-2) = 0
2 + x -6 = 0
x=+4
The oxidation state of sulfur in Sulfurous acid is +4
(c)- Sulfuric acid
The formula of Sulfuric acid isH2SO4. The structure of Sulfuric acid is given below:
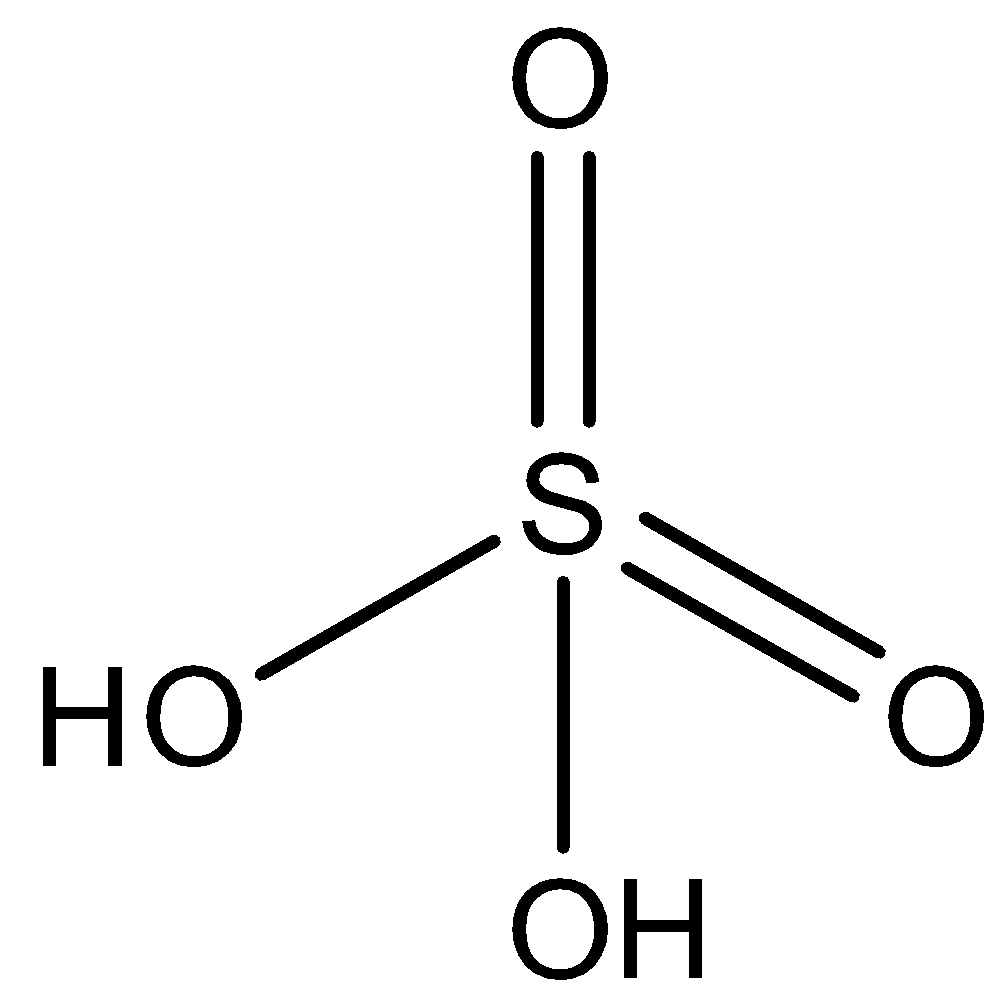
So, to find the oxidation state of sulfur in Sulfuric acid,
Let us assume the oxidation state of sulfur is x and the overall charge of the molecule is zero.
Therefore, 2(+1) + (x) + 4(-2) = 0
2 + x -8 = 0
x=+6
The oxidation state of sulfur in Sulfuric acid is +6.
(d)- Disulfuric acid
The formula of Disulfuric acid is H2S2O7. The structure of Disulfuric acid is given below:
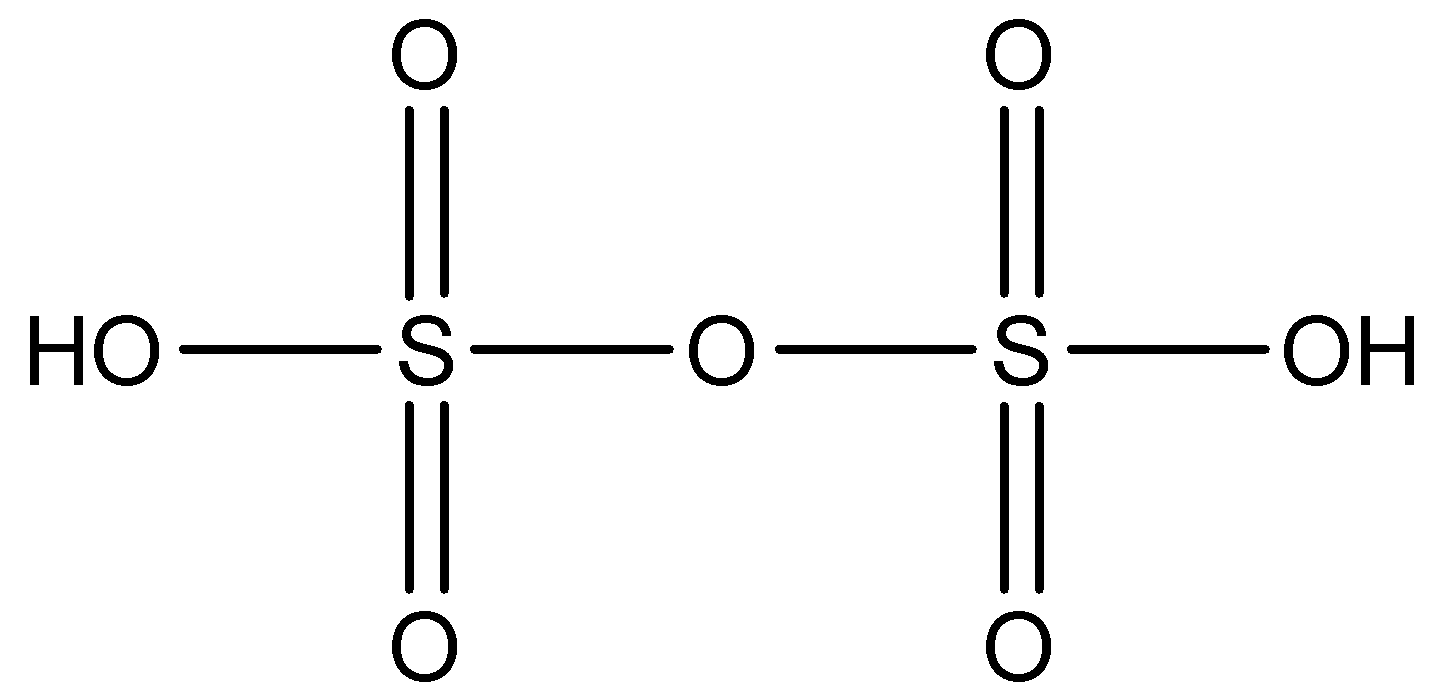
So, to find the oxidation state of sulfur of Disulfuric acid,
Let us assume the oxidation state of sulfur is x and the overall charge of the molecule is zero.
Therefore, 2(+1) + 2(x) + 7(-2) = 0
2 + 2x -14 = 0
x=+6
So, the oxidation state of sulfur in Disulfuric acid is +6.
So, the correct answer is “Option A”.
Note: In all the cases above the overall charge was zero, if the compound has an overall charge other than zero, then the sum of the oxidation state of all the atoms in the molecule is equal to the overall charge of the molecule.
