Question
Question: In which of the following species the central atom has the type of hybridization which is not the sa...
In which of the following species the central atom has the type of hybridization which is not the same as that of the other three.
A) SF4
B) I3−
C) SbCl52−
D) PCl5
Solution
While answering this question we must read the question twice to confirm what is actually asked. They are asking us to select an odd one out in this case. Thus we can go for all the options to see the hybridizations so as to get this question done.
Complete answer:
Hybridization formula can be represented as:
Hybridization=21[V+M−C+A]
Here,
v= number of valence electrons
m=monovalent
c=positive charge
a=negative charge
We will calculate hybridization for all the given compounds thus we can answer this question easily.
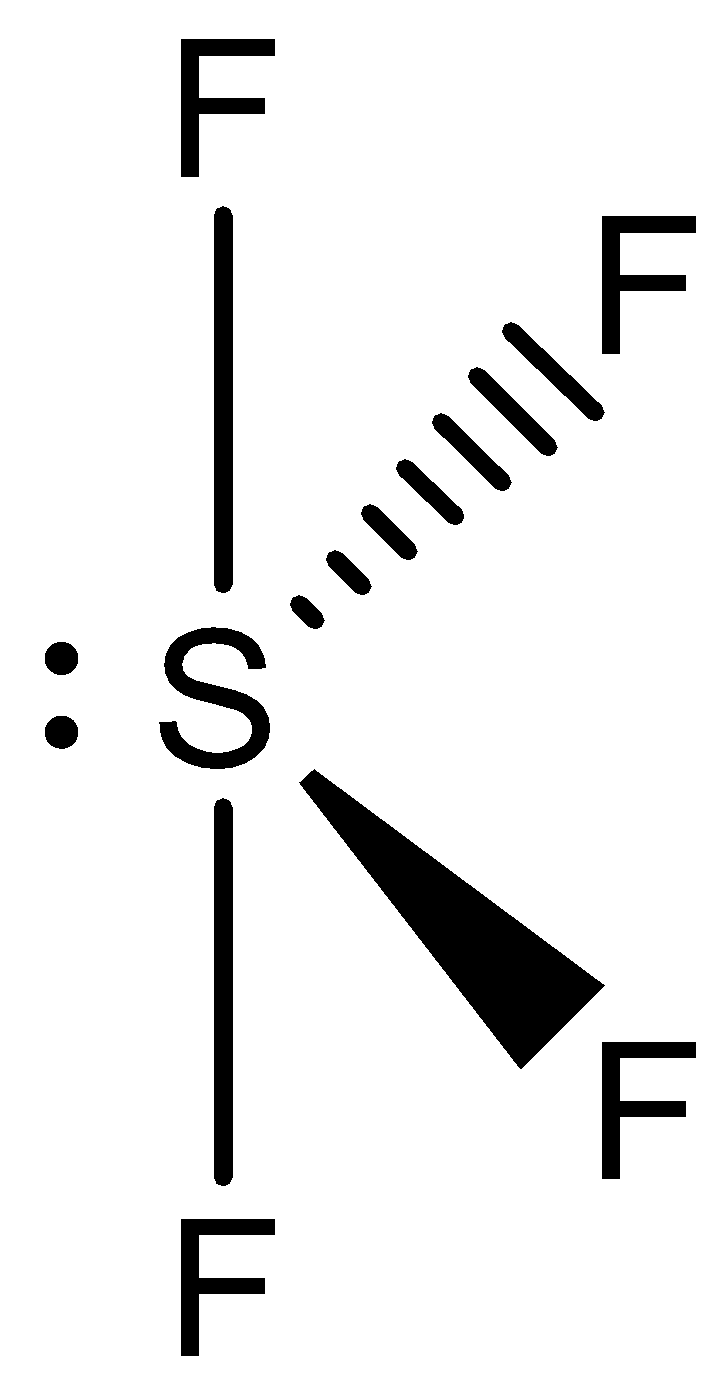
SF4
Hybridization: sp3d
=21(6+4)=5
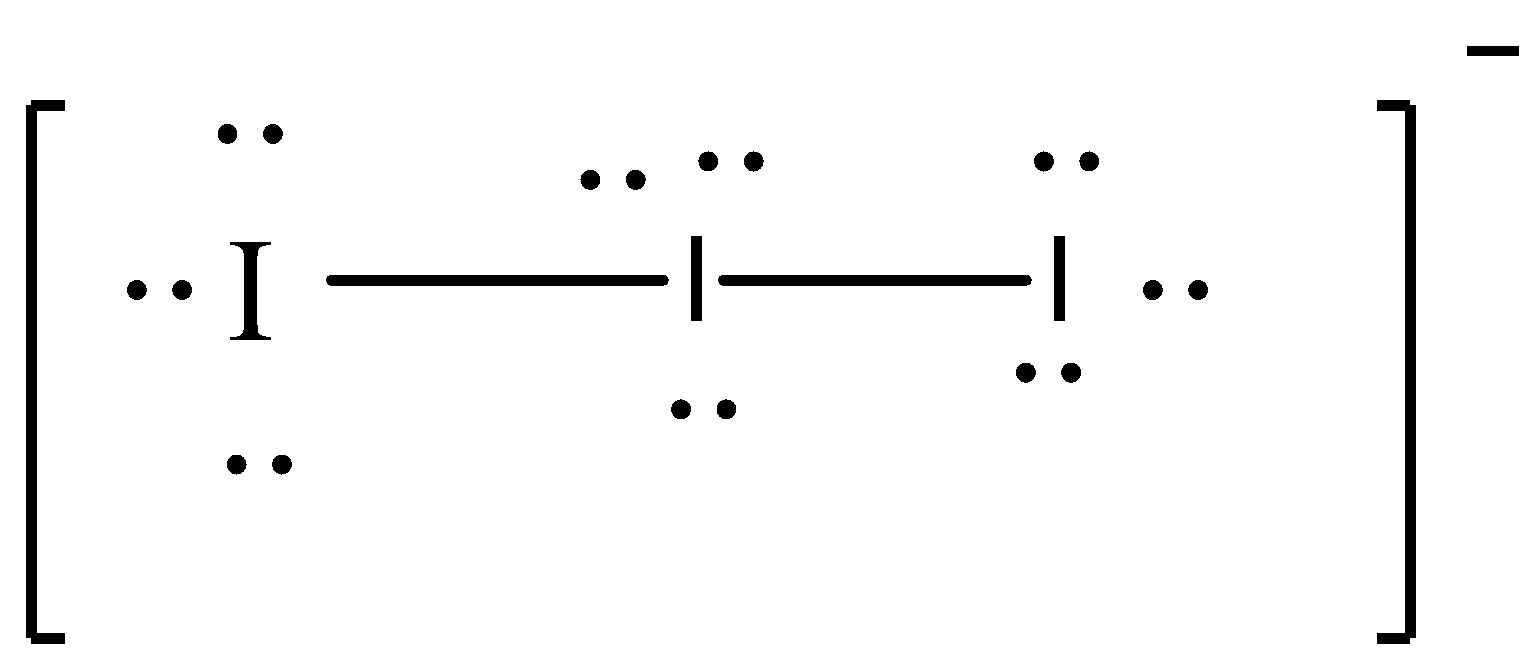
We will calculate hybridization for I3−
Hybridization: sp3d
=21(7+2+1)=5
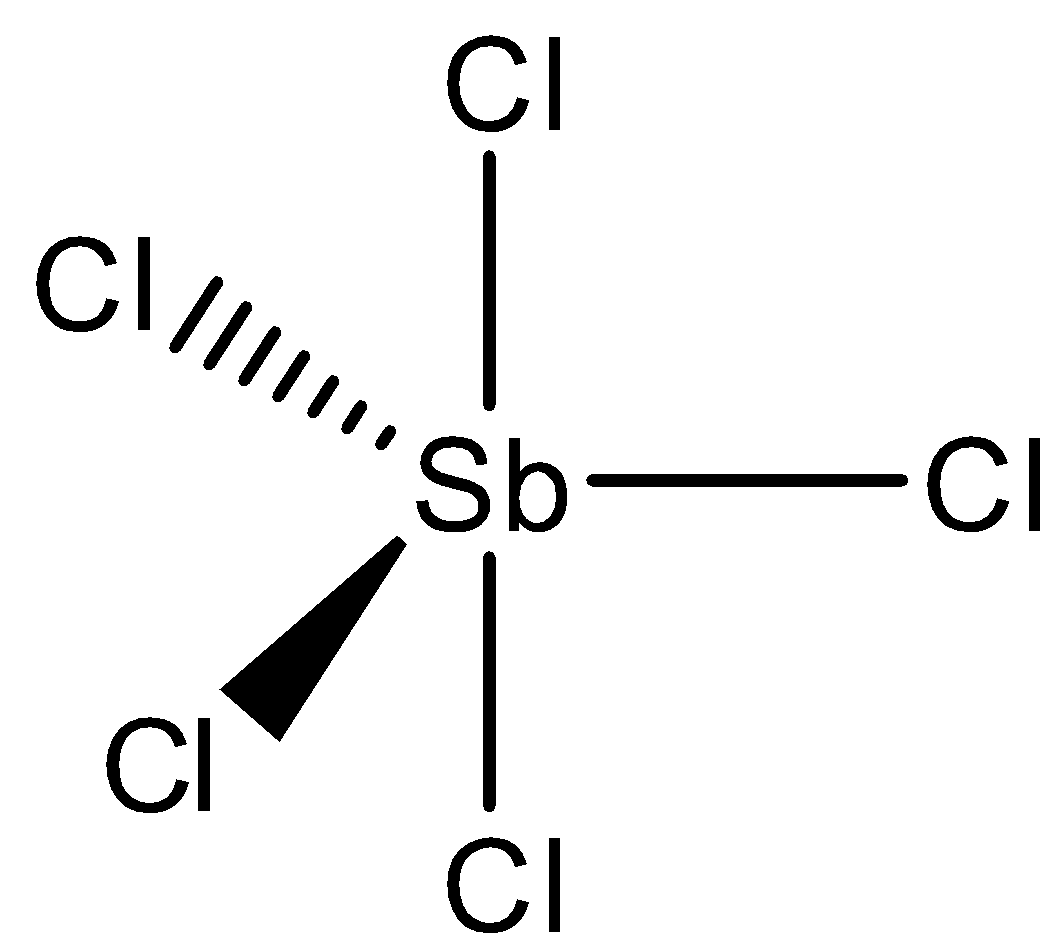
We will calculate hybridization for SbCl52−
Hybridization: sp3d2
=21(5+5+2)=6
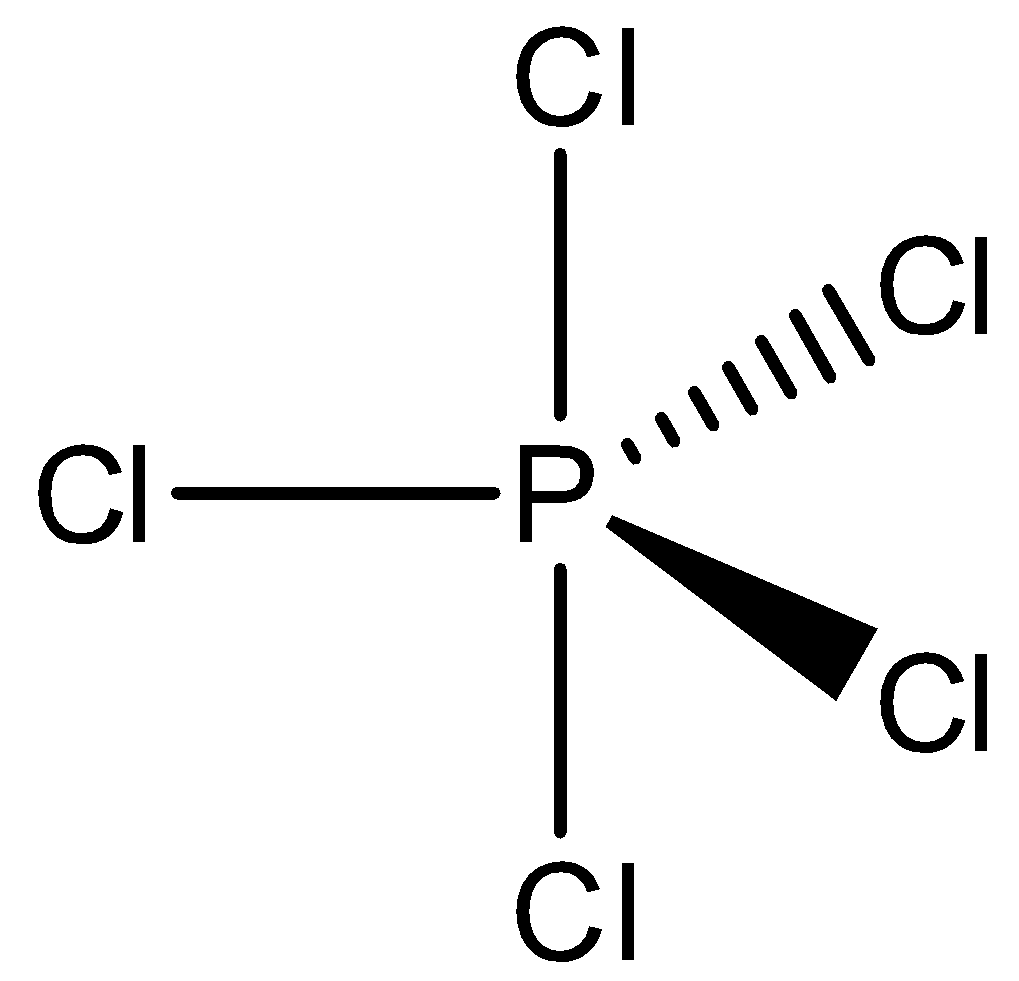
We will calculate hybridization for PCl5
Hybridization: sp3d
=21(5+5)=5
Option A) this is an incorrect option as it has hybridization same as other options as well that is sp3d.
Option b) this is an incorrect option as it has hybridization same as other options as well that is sp3d.
Option C) this option is correct as it has hybridization different from all the other three options which is sp3d2 so according to the asked question this is a correct answer.
Option D) this is an incorrect option as it has hybridization same as other options as well that is sp3d.
Option C is the correct answer.
Note:
We must remember that hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory. The geometry of sp3d hybridization is trigonal bipyramidal whereas for sp3d2 it is octahedral.
