Question
Question: In which of the following species the angle around the central atom is exactly equal to \({{109}^{\c...
In which of the following species the angle around the central atom is exactly equal to 109∘28′
(A) SF4
(B) NH3
(C) NH4+
(D) None of these
Solution
To solve this, find the hybridisation of each of the given molecules. 109∘28′ is shown by a regular tetrahedral species. When the central metal atom is occupied in 4 bond pairs and no lone pairs, it will show a bond angle of 109∘28′.
Complete step by step answer:
We know that in chemistry, the angle between two bonds originating from the same atom in a covalent species is known as the bond angle.
Now let us discuss the relation of hybridisation with the bond angle.
Bond angle 109∘28′ is seen in species with regular tetrahedral geometry and they have a hybridisation of sp3. For this hybridisation, steric number is 4. Steric number is the number of atoms, groups or lone pairs around the central metal atom.
sp3 hybridized molecules may have different shapes and we can decide it by the steric number. For steric number 4, there are 4 structural possibilities but only one includes a bond angle of 109∘28′.
For bond angle to be 109∘28′, there must be 4 bond pairs and no lone pairs.
So now let’s check the molecules given to us and try to find out the number of bond pairs in them.
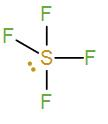
Firstly we have SF4. It has a hybridisation of sp3d (Sulphur has 5 valence electrons. Four will be occupied by the 4 fluorine atoms and the other one will exist as a lone pair.) It will exist as a triangular bipyramidal and the bond angle is not 109∘28′ in it.
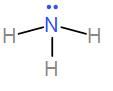
Then we have NH3. Ammonia has three bond pairs and one lone pair and the bond angle is 107∘.
Lastly, we have NH4+. It has 4 bond pairs and no lone pair and it takes up tetrahedral geometry with sp3 hybridisation. Therefore, the bond angle here is 109∘28′.
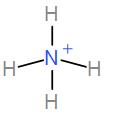
We can see from the above discussion that NH4+ has bond angle of 109∘28′ and this is the required answer.
Therefore, the correct answer is option (C) NH4+.
Note: sp3hybridised molecules can have different shapes and it is decided by its steric number. This hybridisation has a steric number of 4. There are 4 possible structures and they are:
- Central atom occupied in 4 bond pairs will give a bond angle of 109∘28′ .
- Central atoms occupied in 3 bond pairs and 1 lone pair will show a bond angle of 107∘.
- Central atoms occupied in 2 bond pairs and 2 lone pairs will show a bond angle of 104∘5′ .
- Central atoms occupied in 1 bond pair and 3 lone pairs will not have any defined bond angle as there is only one bond.
