Question
Question: In which of the following species S-atom assumes \(s{{p}^{3}}\)-hybrid state? (I)- \(S{{O}_{3}}\) ...
In which of the following species S-atom assumes sp3-hybrid state?
(I)- SO3
(II)- H2S
(III)- SO2
(IV)- S8
(a)- (I) and (II)
(b)- (II) and (III)
(c)- (II) and (IV)
(d)- (III) and (IV)
Solution
In the compound, if the central atom should have sp3-hybrid state, then all the bonds in the compound must be a single bond. If the compound has a double or triple bond then the central atom cannot have sp3-hybrid state. If a single bond is not present, then the lone pair also contributes to sp3-hybrid state.
Complete step by step solution:
In the compound, if the central atom should have sp3-hybrid state, then all the bonds in the compound must be a single bond. If the compound has a double or triple bond then the central atom cannot have sp3-hybrid state. If a single bond is not present, then the lone pair also contributes to sp3-hybrid state.
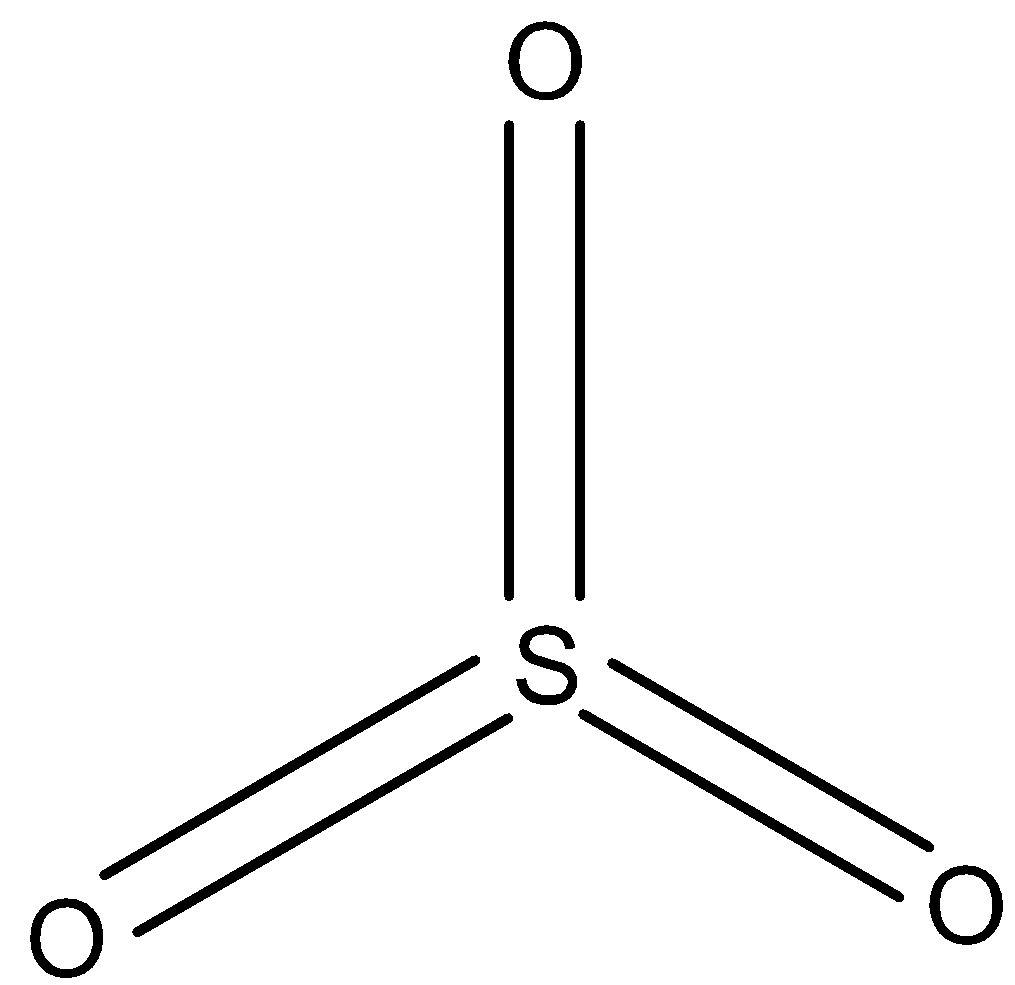
In SO3, all the sulfur atoms are joined to oxygen atoms through a double bond as shown below. So the hybridization of sulfur in SO3 is sp2.
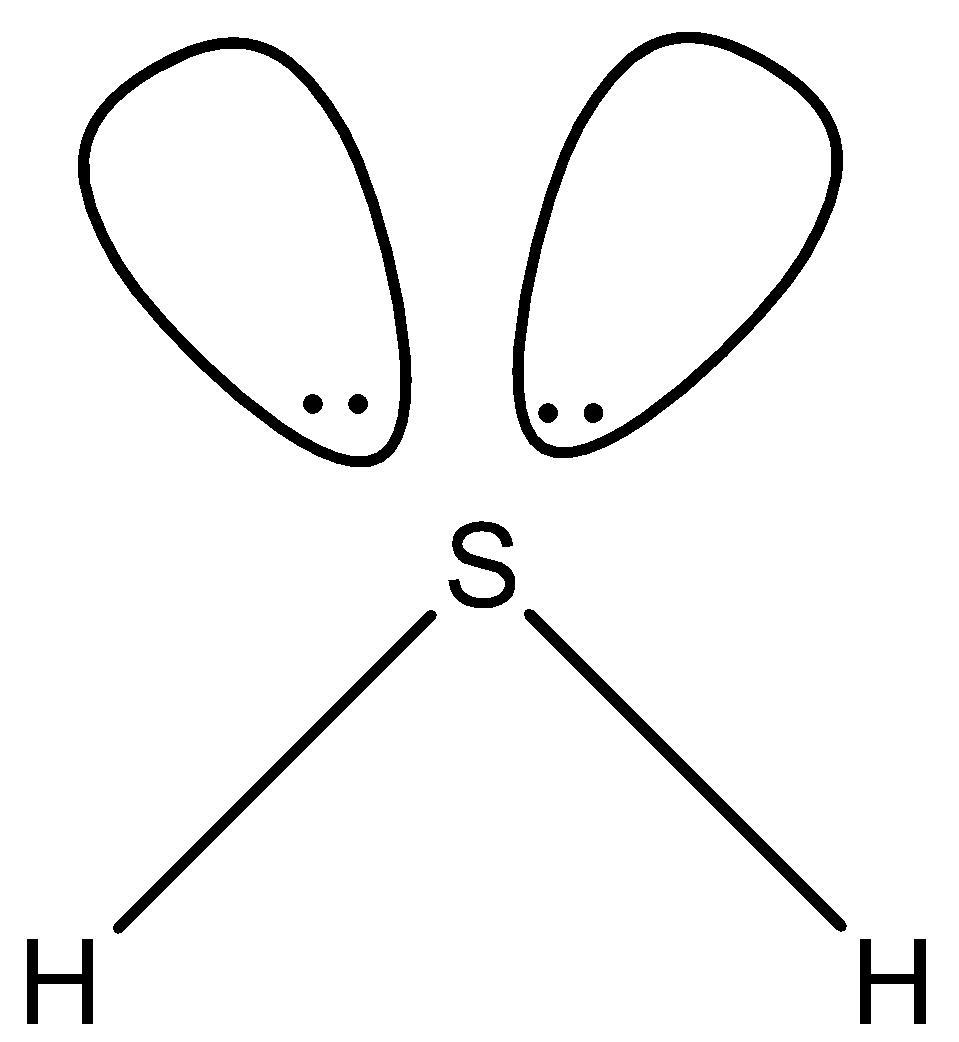
In H2S, there are two single bonds and there are two lone pairs as shown below. So the hybridization of sulfur in H2S is sp3.
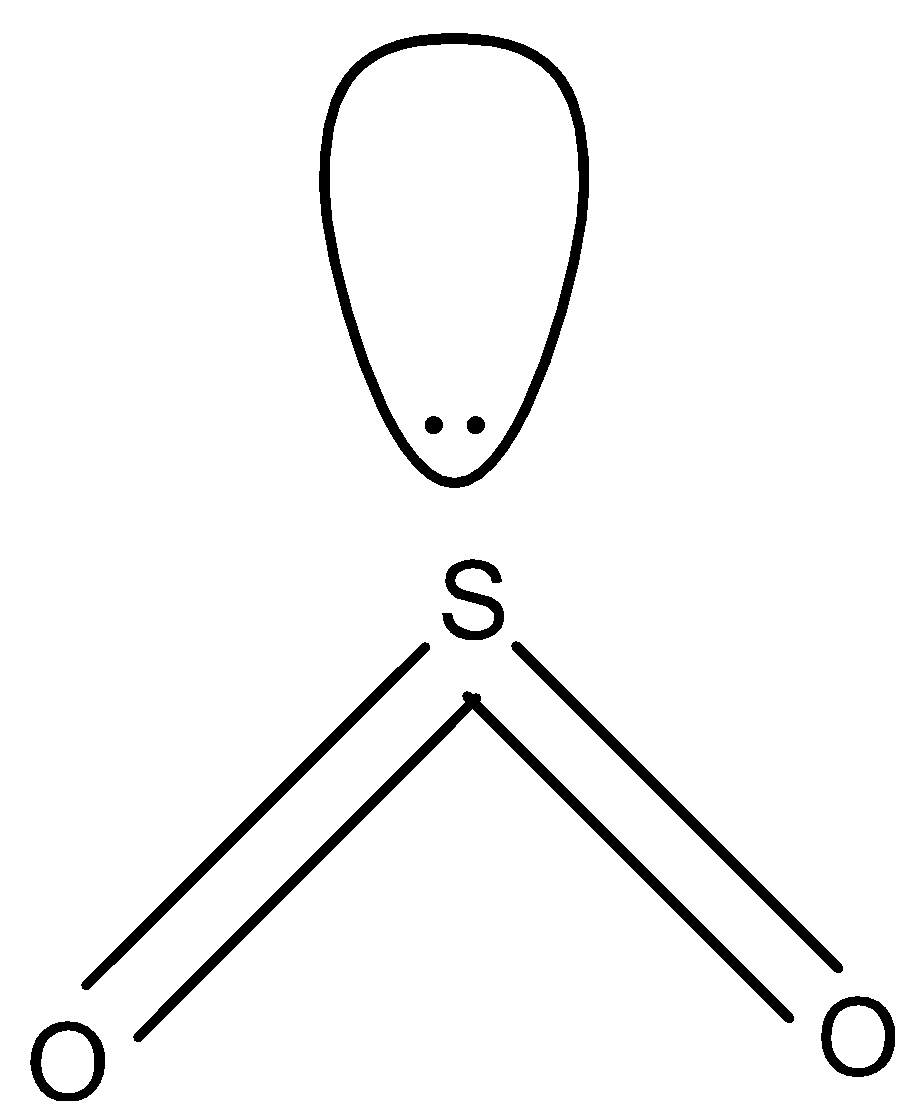
In SO2, there are double bonds and there is one lone pair as shown below. So, the hybridization of sulfur in SO2 is sp2.
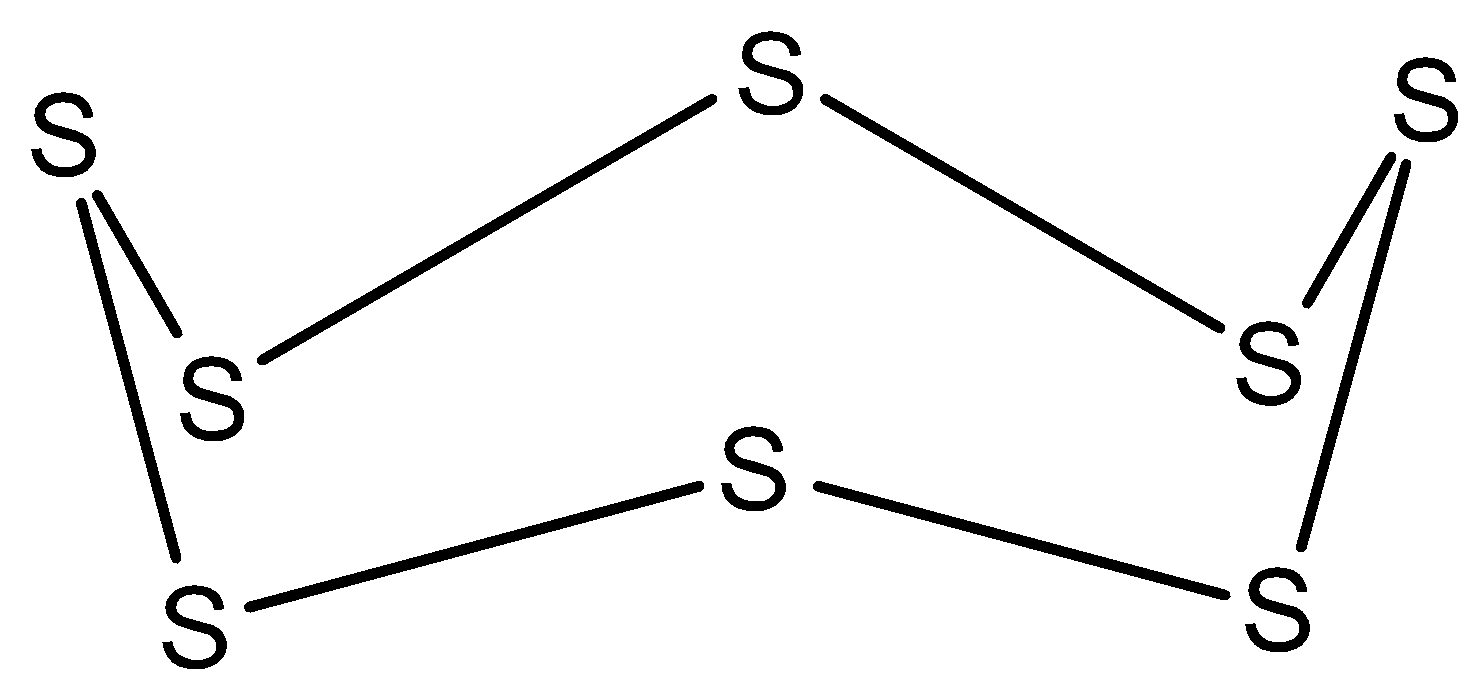
In S8, all the bonds in this molecule are single as shown below. So, the hybridization of sulfur in S8 is sp3.
So, in compound (II) and (IV) the hybrid state is sp3.
So, the correct answer is “Option C”.
Note: If the compound is having a double bond then the hybrid state will be sp2 and if the compound is having a triple bond then the hybrid state will be sp.
