Question
Question: In which of the following reagents, amino benzene is soluble? A) \(HCl\) B) \(NaOH\) C) \(N{H...
In which of the following reagents, amino benzene is soluble?
A) HCl
B) NaOH
C) NH3
D) NaHCO3
Solution
We must have to remember that aminobenzene is most commonly known as Aniline having a benzene structure and an amine group present in it. Structure of amino benzene or aniline can be represented as:
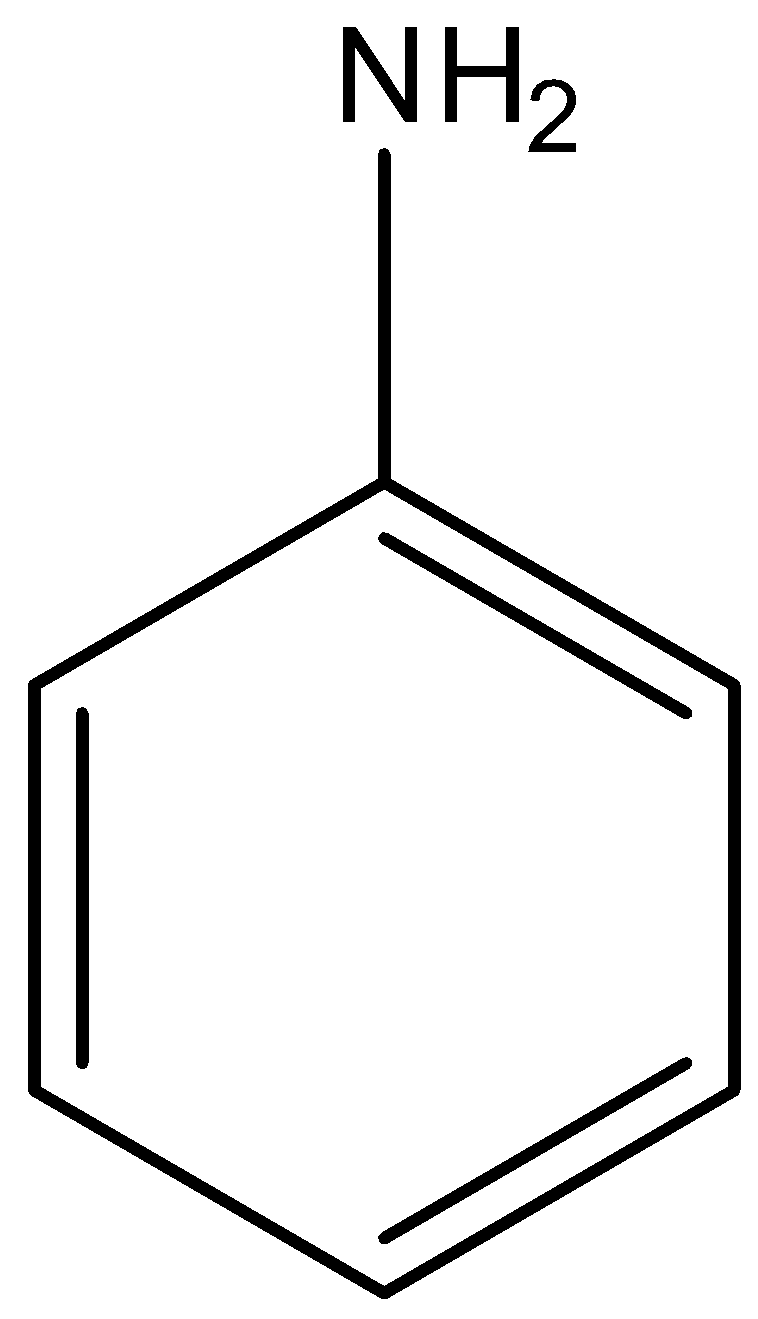
Complete answer:
Now we look for the solubility of aniline in the different solvents given as options. We will look at all the options to answer this question:
Option A) this is a correct option as amino benzene is soluble in hydrochloric acid. The reaction can be represented as:
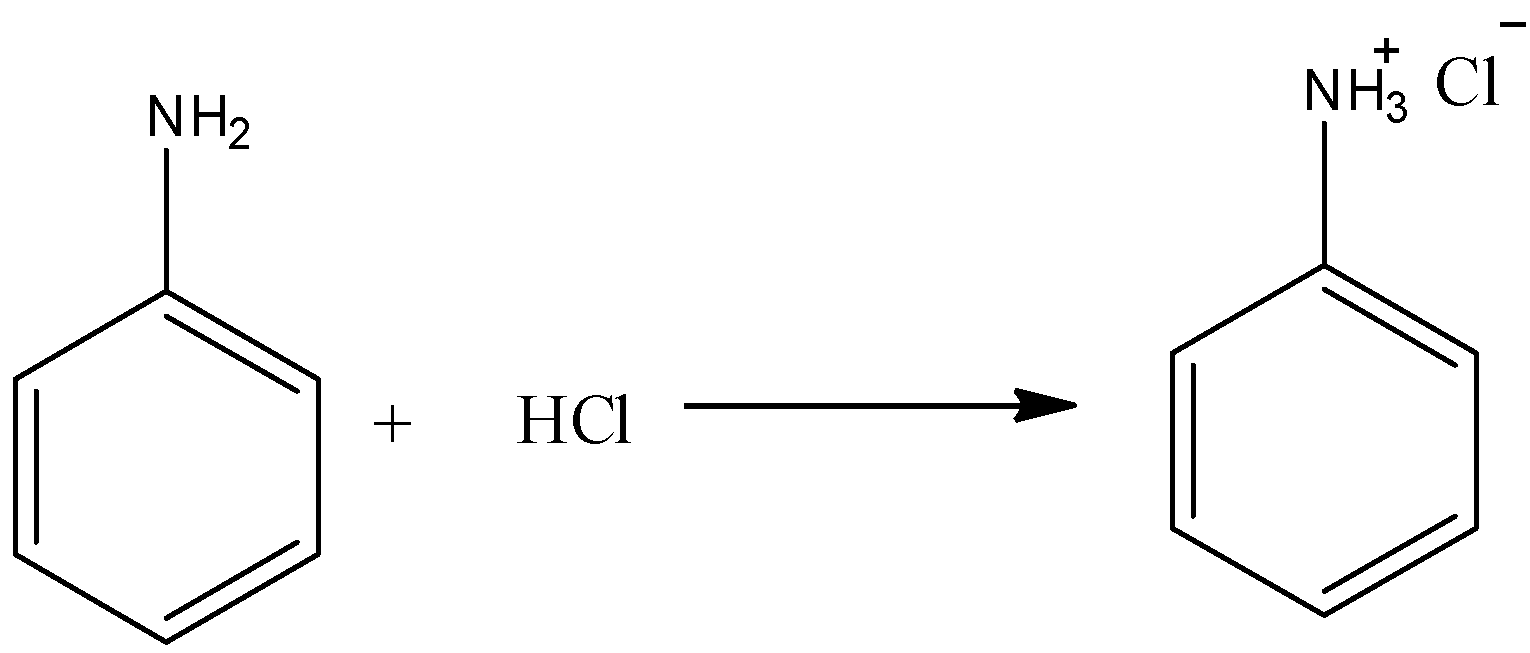
Aniline is a colorless liquid which is sparingly soluble in water. But when aniline is dissolved with hydrochloric acid it produces an anilinium chloride salt which is also water soluble. Thus we can say that aniline is soluble in hydrochloric acid.
Option B) this option is incorrect as sodium hydroxide is a base which makes aniline slightly polar in nature thus on having weak polarity they will have less interaction between ions thus it is insoluble.
Option C) this is an incorrect option.
Option D) this is an incorrect option as Benzoic acid is a weak acid and will react with a strong base to form an anion which will dissolve in water. Aniline is a weak base and will be deprotonated by reaction with a strong acid to form a water soluble cation.
Note:
We must have to know that aniline is a weak base and is considered to form salts with mineral acids. Aniline is slightly soluble in water and mixes readily with most organic solvents. It is used to make a wide variety of products such as polyurethane foam, agricultural chemicals, synthetic dyes, antioxidants, stabilizers for the rubber industry, herbicides, varnishes and explosives.
