Question
Question: In which of the following reactions only a single isomer of alkene is formed on reaction with KOH an...
In which of the following reactions only a single isomer of alkene is formed on reaction with KOH and ethanol?
(a)- 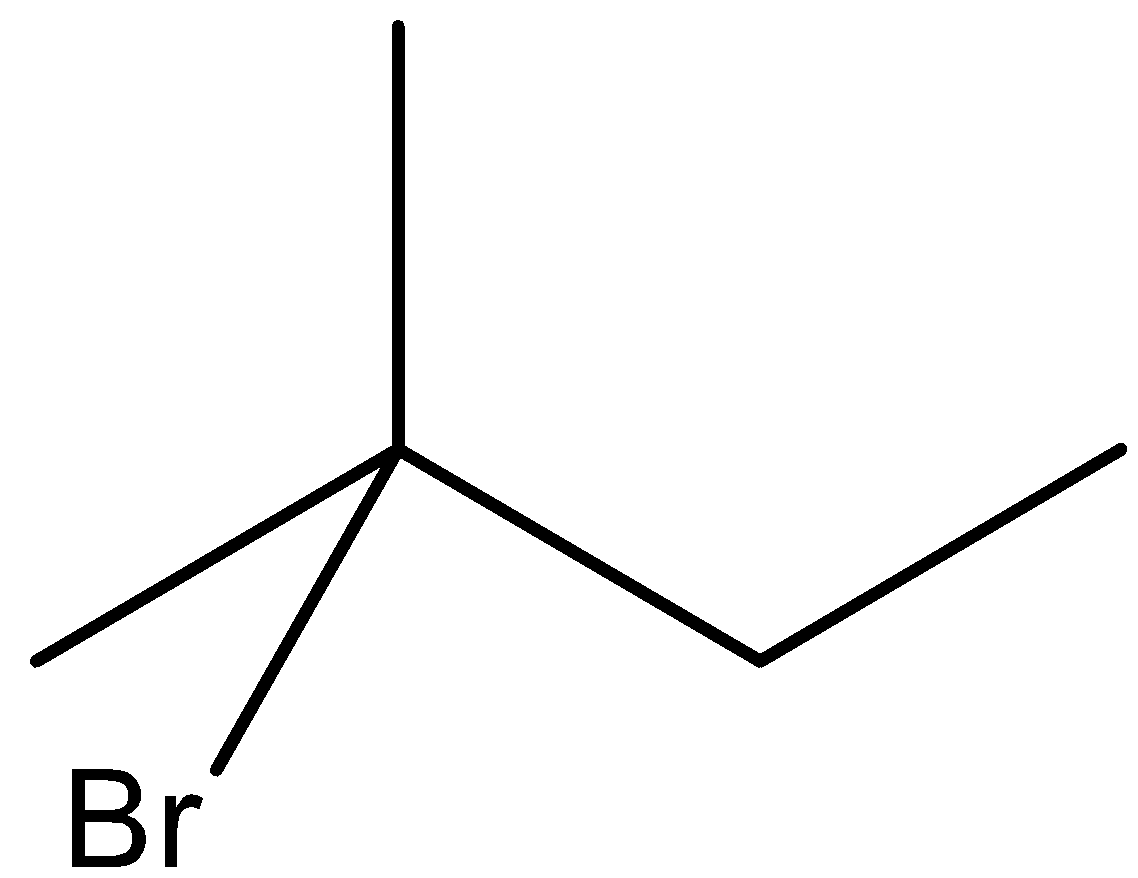
(b)- C6H5−CH2−CH2−CH2−Br
(c)- C6H5−CH2−CH∣Br−C6H5
(d)- 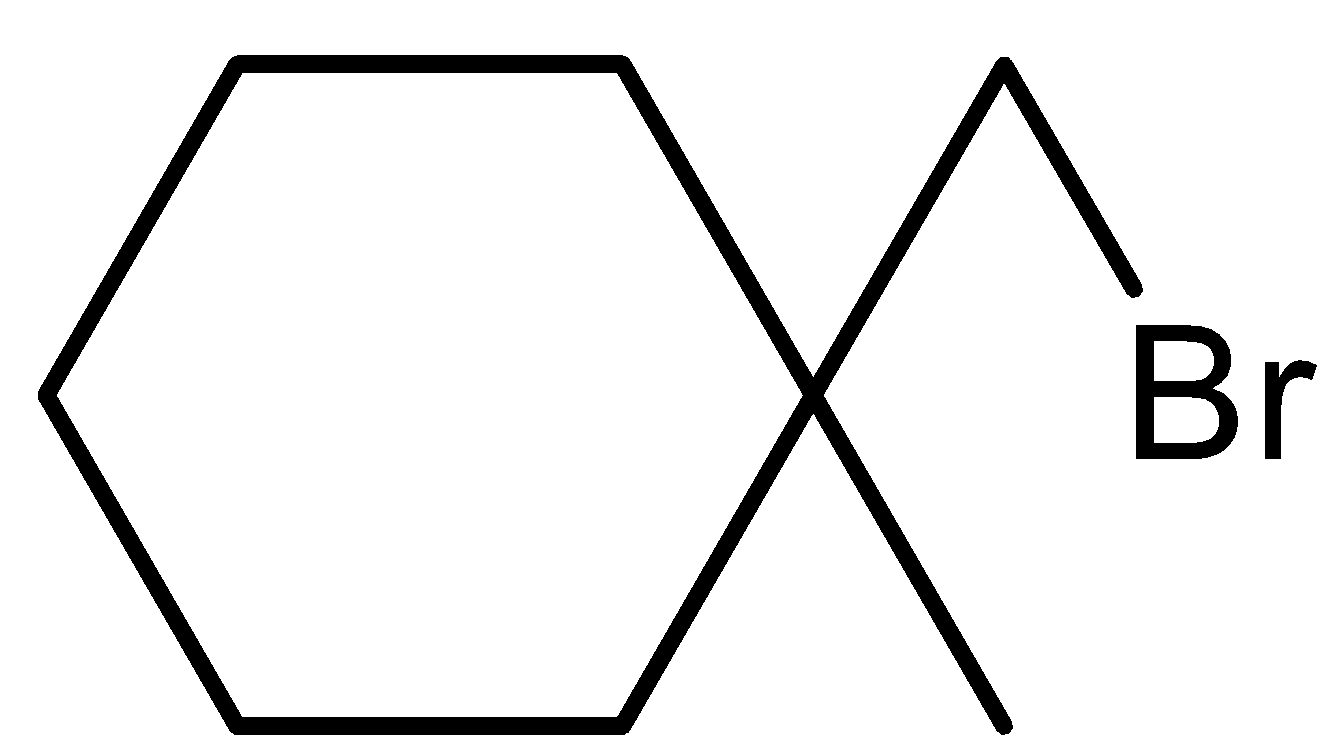
Solution
In all the compounds, a bromine atom is present on the carbon chain, which means all are alkyl or aryl halides. When the alkyl or aryl halide reacts with KOH and ethanol, there will be the formation of an alkene by the elimination of bromine atoms and a hydrogen atom from the adjacent carbon atom.
Complete answer:
In all the compounds, bromine atoms are present on the carbon chain, which means all are alkyl or aryl halides. When the alkyl or aryl halide reacts with KOH and ethanol, there will be the formation of an alkene by the elimination of bromine atoms and a hydrogen atom from the adjacent carbon atom.
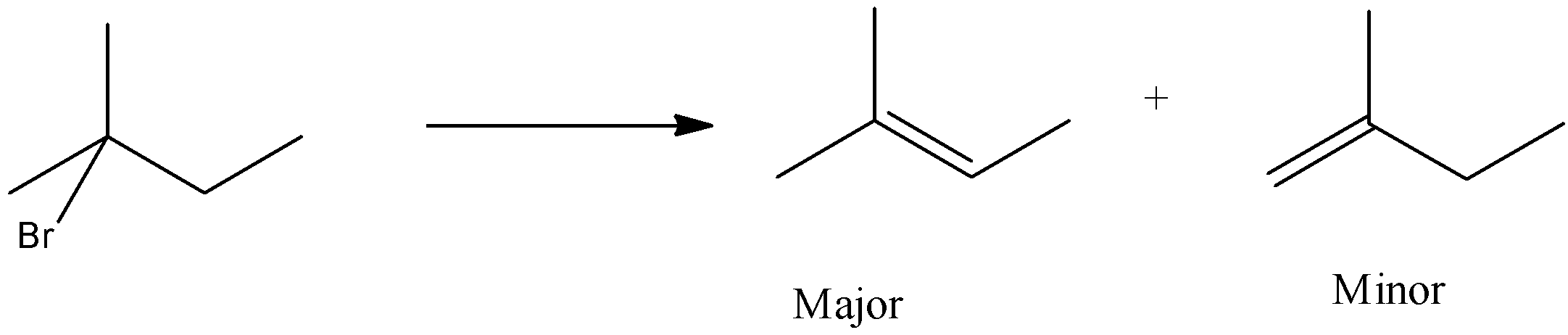
In option (a), the bromine is present on the second carbon atom, when it reacts with KOH and ethanol, then one product will be formed in which the hydrogen atom will be removed from the third carbon atom and the other from the first carbon atom.
In option (b), the bromine is present only on the terminal carbon atom, so the hydrogen atom will be removed from the carbon atom attached with the bromine atom.
C6H5−CH2−CH2−CH2−BrKOHethanolC6H5−CH2−CH2=CH
In option (c), the bromine is present on the carbon atom between the phenyl groups, when it reacts with KOH and ethanol, then one product will be formed in which the hydrogen atom will be removed from the third carbon atom and the other from the first carbon atom.
C6H5−CH2−CH∣Br−C6H5KOHethanolC6H5−CH2=CH−C6H5+C6H5−CH2−CH=C6H5
In option (d), there will be no reaction because there is no hydrogen atom on the carbon atom that contains the bromine atom.
Therefore, the correct answer is an option (b).
Note:
The reaction of an alkyl or aryl halide with the KOH and ethanol, then there is the formation of an alkene or when the alkyl or aryl halide reacts with KOH solution then there is the formation of alcohol.
