Question
Question: In which of the following reaction formation of racemic mixture- A. 
B.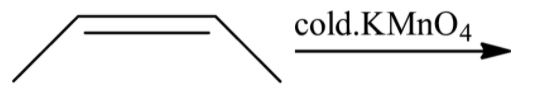
C.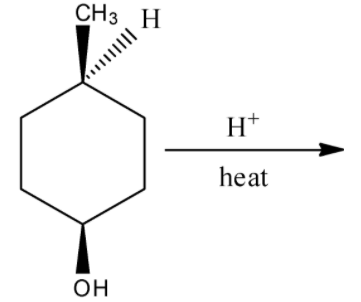
D.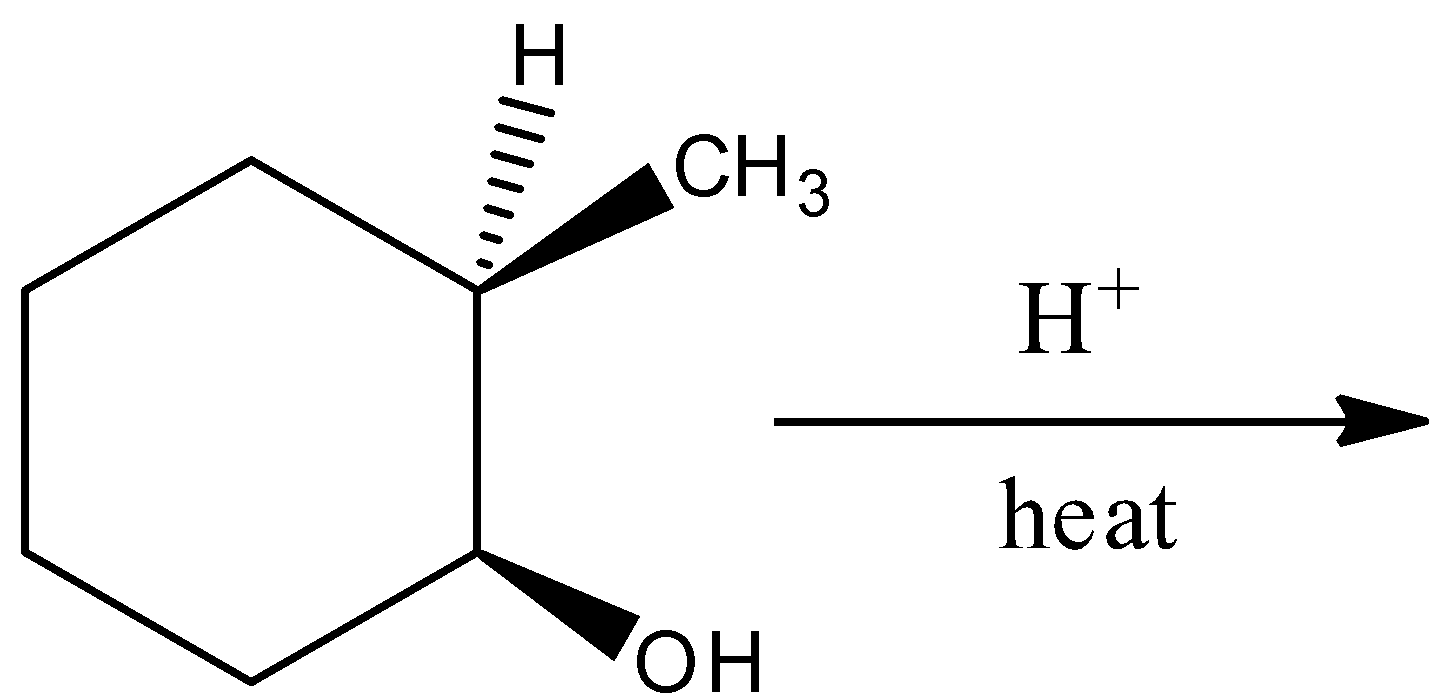
Solution
We need to know that the racemic mixture is also known as racemate. In a racemic mixture, the chiral molecule contains the left and right enantiomer in equal amounts. Hence, the mixture has the mirror image of one another and it is optically inactive. The racemic acid is the first known racemic mixture and it is the mixture of two enantiomeric isomers of the tartaric acid.
Complete answer:
Here the given reactant is alkene. When (E)-but-2-ene is reacted with bromine and carbon tetrachloride and there is a formation of racemic mixture. And it shows two enantiomeric isomers. Let’s see the reaction,
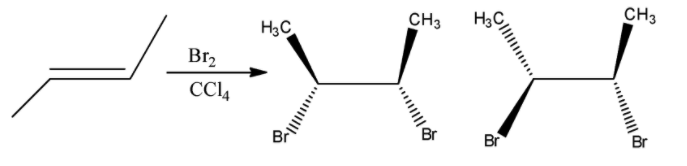
Hence, option (A) is correct.
When (Z)-but-2-ene is reacted with potassium permanganate solution, there will not be a formation of enantiomeric mixture. Hence, the option (B) is incorrect.
There will not a formation of enantiomeric isomer by the hydration reaction of (1s,4s)-4-methylcyclohexan-1-ol in the presence of heat. Hence, option (C) is incorrect.
By the hydration reaction of (1S,2R)-2-methylcyclohexan-1-ol in the presence of heat;, there will not form a racemic mixture. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We must have to know that the racemic mixture contains the chiral molecules and it does not shows the optical activity. Thus, the net optical activity is zero. The formation of racemic mixture from chiral material is known as racemization. And forms, same amount of enantiomeric substances. And for example, formation of racemic tartaric acid by the reaction of d- tartaric acid and l- tartaric acid.
