Question
Question: In which of the following properties, the two enantiomers of lactic acid differ from each other? A...
In which of the following properties, the two enantiomers of lactic acid differ from each other?
A.Sign of specific rotation
B.Density
C.Melting point
D.Refractive index
Solution
As we know that enantiomers are stereoisomers of a compound which are related to one another as non-super imposable mirror images. They have different dimensional arrangements of equivalent atoms/ groups in a molecule.
Complete step by step answer:
As we know if a carbon has four different substituent then this carbon is said to be chiral carbon. The structural property is termed as enantiomers which have the same density, refractive index. Increasing the number of possible geometric forms is due to the multiple chiral.
Now we can draw the enantiomers of lactic acid as,
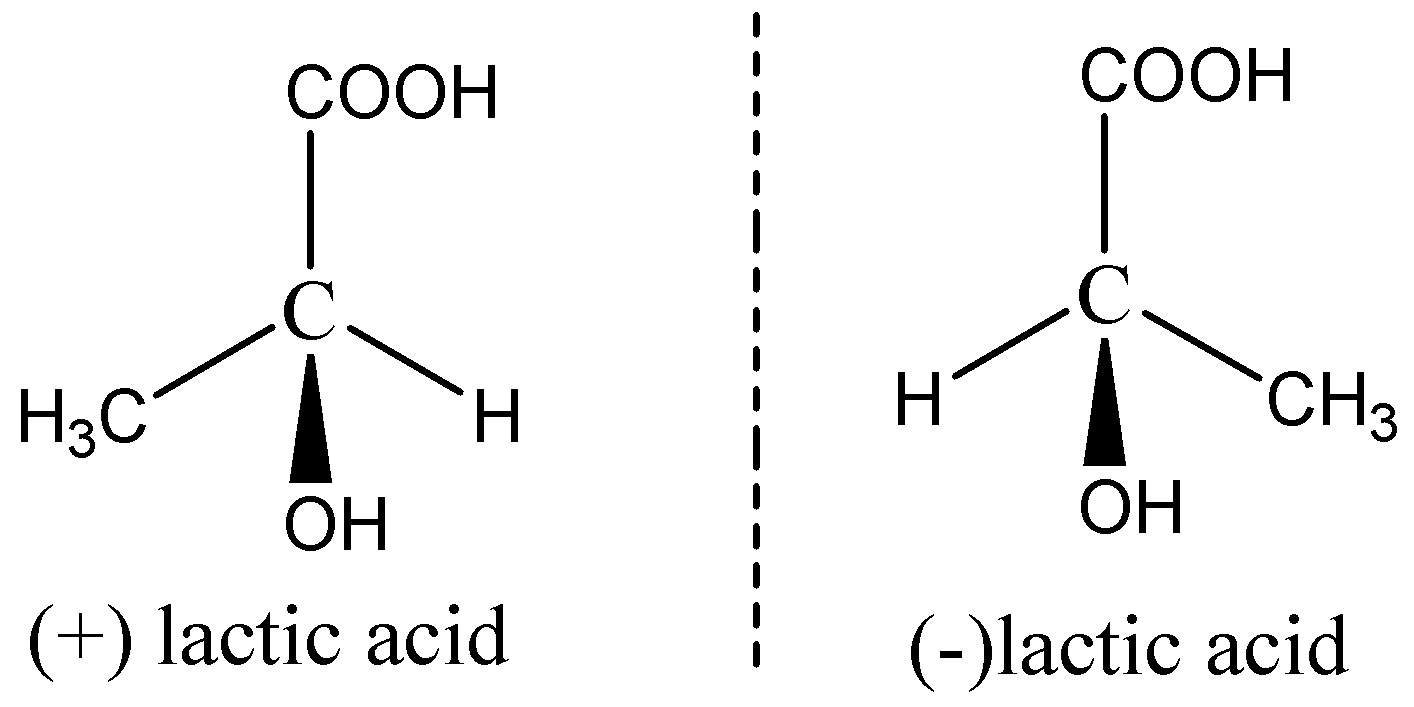
(+) lactic acid (-) lactic acid are non-superimposable mirror images of every other.
The two enantiomers differ in the sign of rotation.
Therefore, the option A is correct.
The both enantiomers don't differ in density.
Therefore, option B is wrong.
Melting point doesn’t attack much in their two enantiomers difference.
Therefore, option C is wrong.
Refractive index doesn’t involve difference.
Therefore, the option D is wrong.
The two enantiomers differ from each other by sign of specific rotation, both are having opposite signs of specific rotation.
So, the correct answer is Option A.
Additional Information:
Enantiomers: One of the enantiomers rotates the plane of polarization to the left is called laevorotatory while the other one rotates the plane of polarization to the right is called dextrorotatory. We can find the number of enantiomers in any compound by using the formula 2n.
Where,
n=number of the chiral carbon.
In case of lactic acid, the no. of chiral carbon is 1.
2n=21=2
So, there are two enantiomers of lactic acid.
Note:
We must remember that the enantiomers are equal in all their physical properties except for their optical rotation, as they rotate the plane of polarised light by equal amounts in the opposite direction.
