Question
Question: In which of the following pairs, the two species are iso-structural? A. \[BrO_3^ - \] and \[Xe{O_3...
In which of the following pairs, the two species are iso-structural?
A. BrO3− and XeO3
B. SF4 and XeF4
C. SO32− and NO3−
D. BF3 and NF3
Solution
Iso-structural is defined as those species which contain the same number of atoms arranged in the same structural form which same number of chemical bonds and lone pairs of electrons..
Complete step by step answer:
A. BrO3− and XeO3
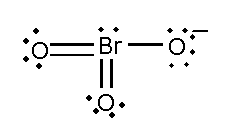
In this compound, the total number of atoms present in this structure is 4, lone pair present is 7, minus charge shows that it contains extra electrons.
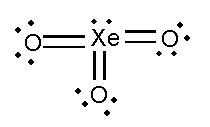
In this compound, the total number of atoms present in this structure is 4, lone pair present is 7.
Both the given structures are isostructural to each other.
B. SF4 and XeF4
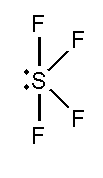
In this compound, the total number of atoms present in this structure is 4, lone pair present is 1.
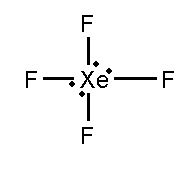
In this compound, the total number of atoms present in this structure is 4, lone pair present is 2.
Both the given compounds are not isostructural to each other.
C. SO32− and NO3−
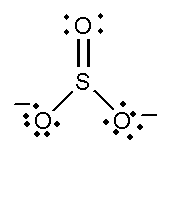
In this compound, the total number of atoms present in this structure is 4, lone pair present is 8. Two single bonds and one double bond is present. The total charge is -2.
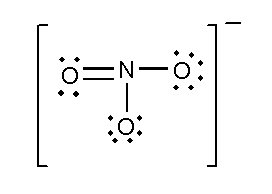
In this compound, the total number of atoms present in this structure is 4, lone pair present is 8. Two single bonds and one double bond is present. The total charge is -1.
Both the given compounds are not isostructural to each other.
D. D. BF3 and NF3
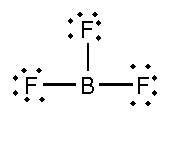
In this compound, the total number of atoms present is 4, the total number of lone pairs present is 9 and three single bonds are present.
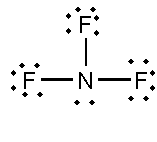
In this compound, the total number of atoms present is 4, the total number of lone pairs present is 10 and three single bonds are present.
Both the given compounds are not isostructural to each other.
So, the correct answer is Option A.
Note: The correct option can also be found by the formula of hybridization which is shown below.
⇒21[noofvalencee−oncentralatom+noofmonovalentatom±charge]
For BrO3−
⇒21[7+0+1]=4sp3
For XeO3
⇒21[8+0±0]=4sp3
