Question
Question: In which of the following option(s) all species contain \(X-O-X\) bond(s) in structure (X= central a...
In which of the following option(s) all species contain X−O−X bond(s) in structure (X= central atom)? (This question has multiple correct options).
(a)- H2S2O5, S3O9, S2O62−
(b)- P4O10, P4O6, H5P3O10
(c)- N2O5, N2O, N2O4
(d)- S3O9, P4O6, Si2O76−
Solution
All the compounds given in the above option are oxoacids of sulfur, phosphorus, nitrogen, and silicon. Since oxoacids are made up of many elements, X−O−X bonds are formed easily.
Complete step by step answer:
All the compounds given in the above options are oxoacids of sulfur, phosphorus, nitrogen, and silicon. Let us see the structure of all the compounds in the given options.
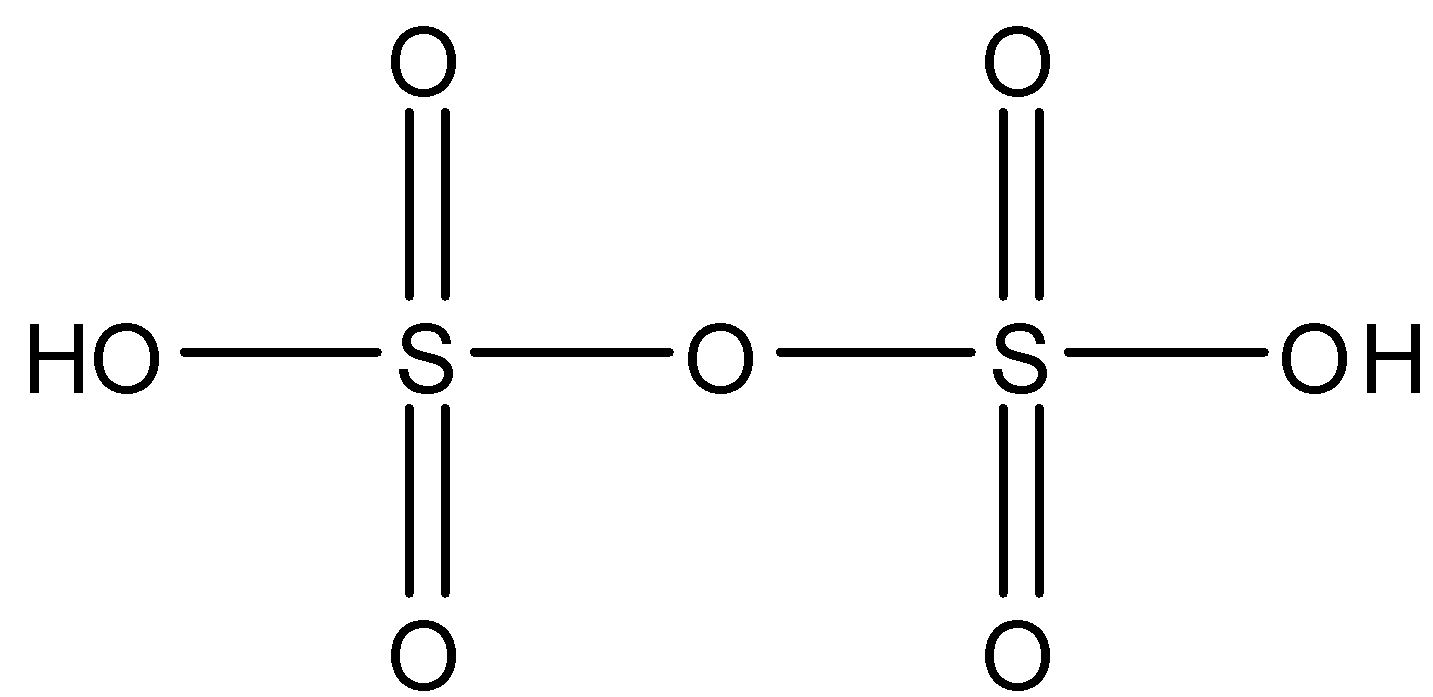
H2S2O5: This compound is known as Disulfurous acid. In this compound, two sulfur atoms are joined together with a single bond. There are three double bond oxygen and two OH bonds. The structure is:
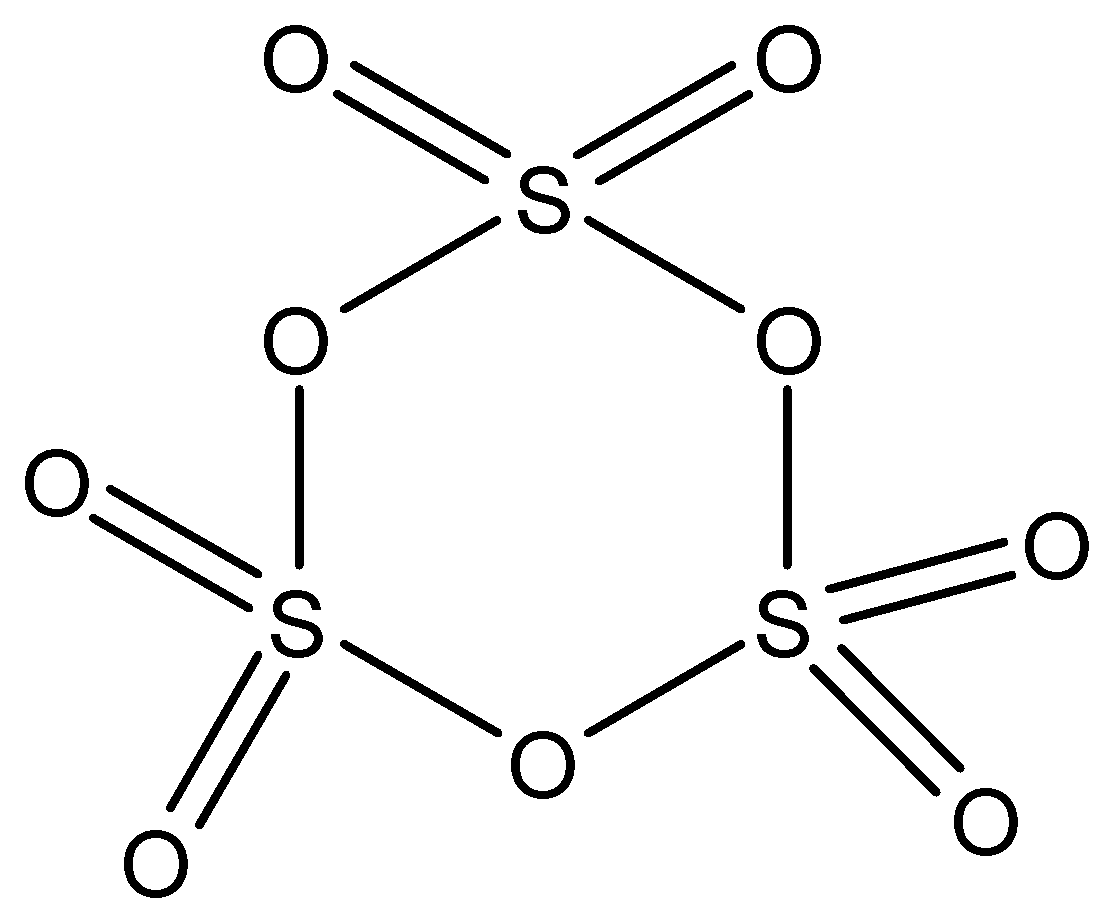
S3O9: This compound is known as sulfur trioxide (orth). This compound has three S−O−S bonds and each sulfur atom is further attached to two oxygen atoms by a double bond. The structure is:
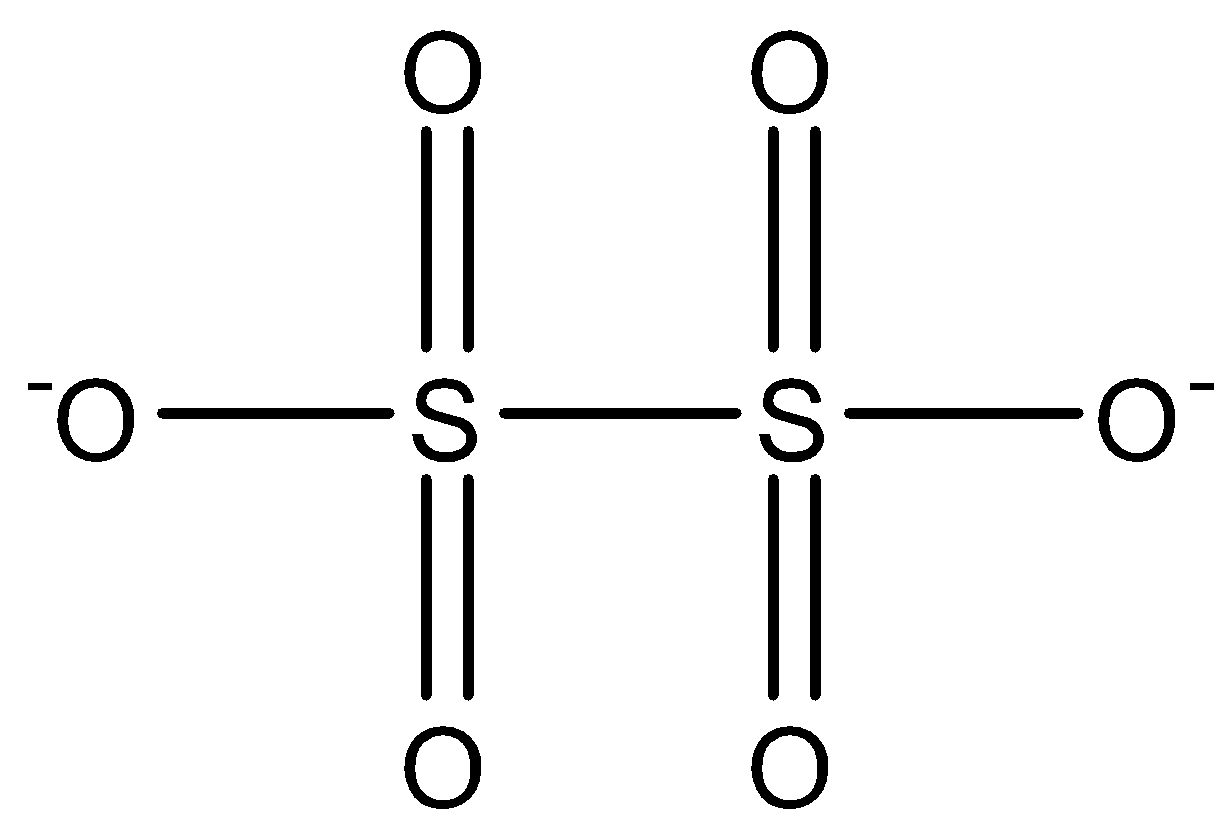
S2O62−: This compound is known as the conjugate acid of Dithionic acid. In this compound, two sulfur atoms are joined together with a single bond. Both the sulfur is attached to two oxygen atoms with a double bond and one oxygen atom with a single bond has a negative charge. The structure is:
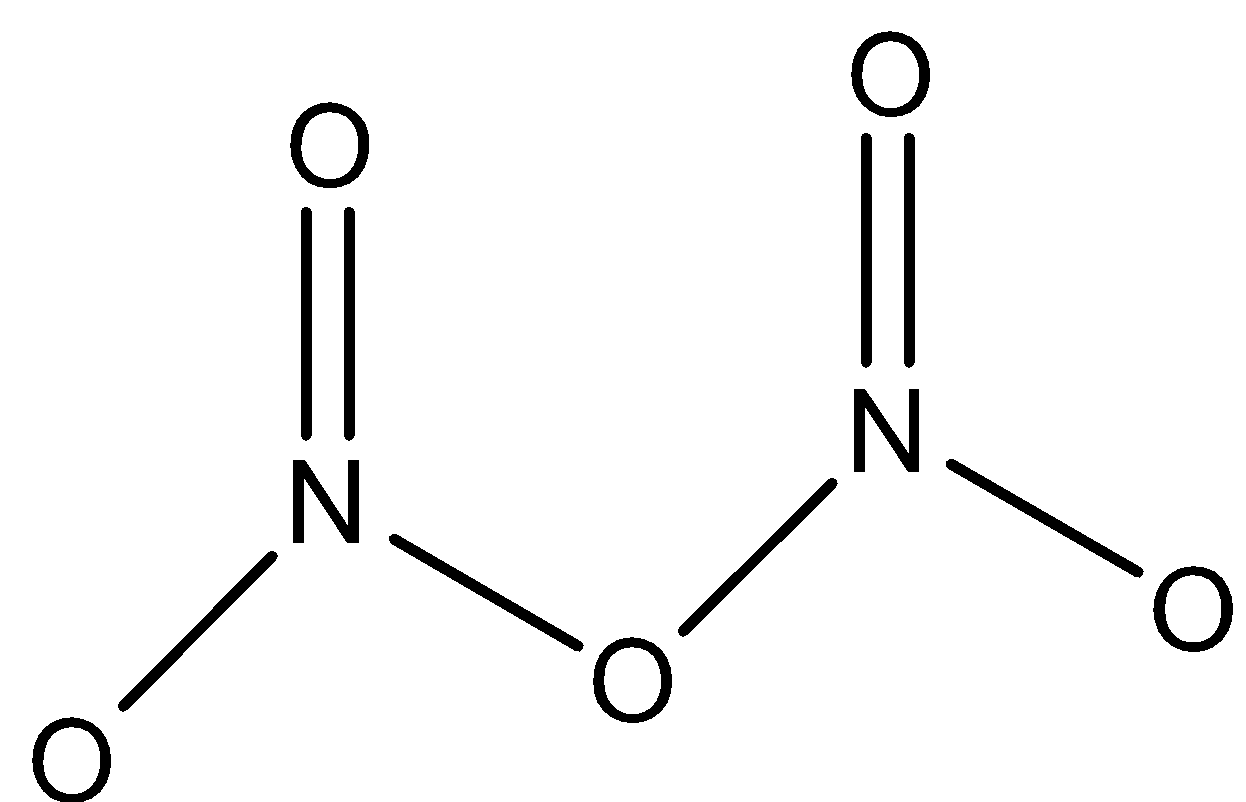
N2O5: This compound is known as dinitrogen pentoxide. This compound has one N−O−N bond. And both nitrogen atoms are joined to two oxygen atoms. The structure is:
N2O: This compound is known as nitrous oxide. The structure is given below:

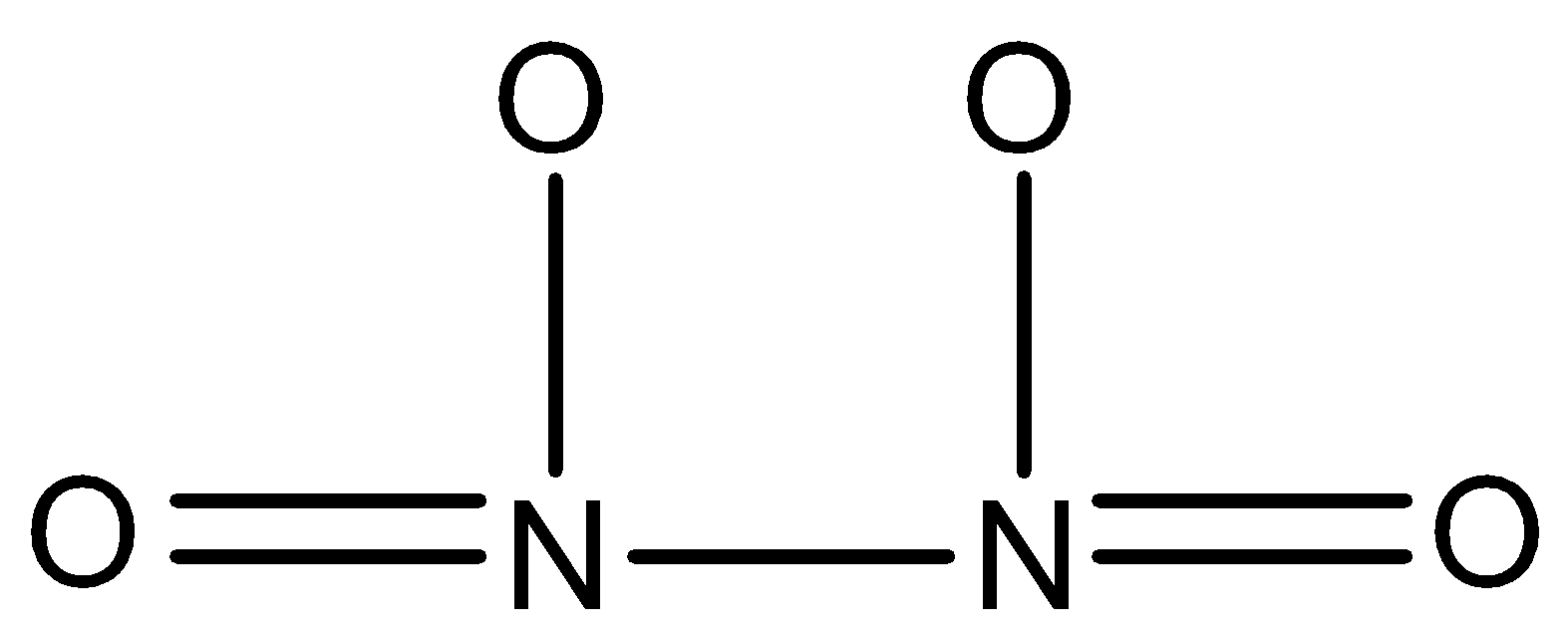
N2O4: This compound is known as dinitrogen tetroxide. In this, two nitrogen atoms are joined together by a single bond and both nitrogen atoms are joined to two oxygen atoms. The structure is:
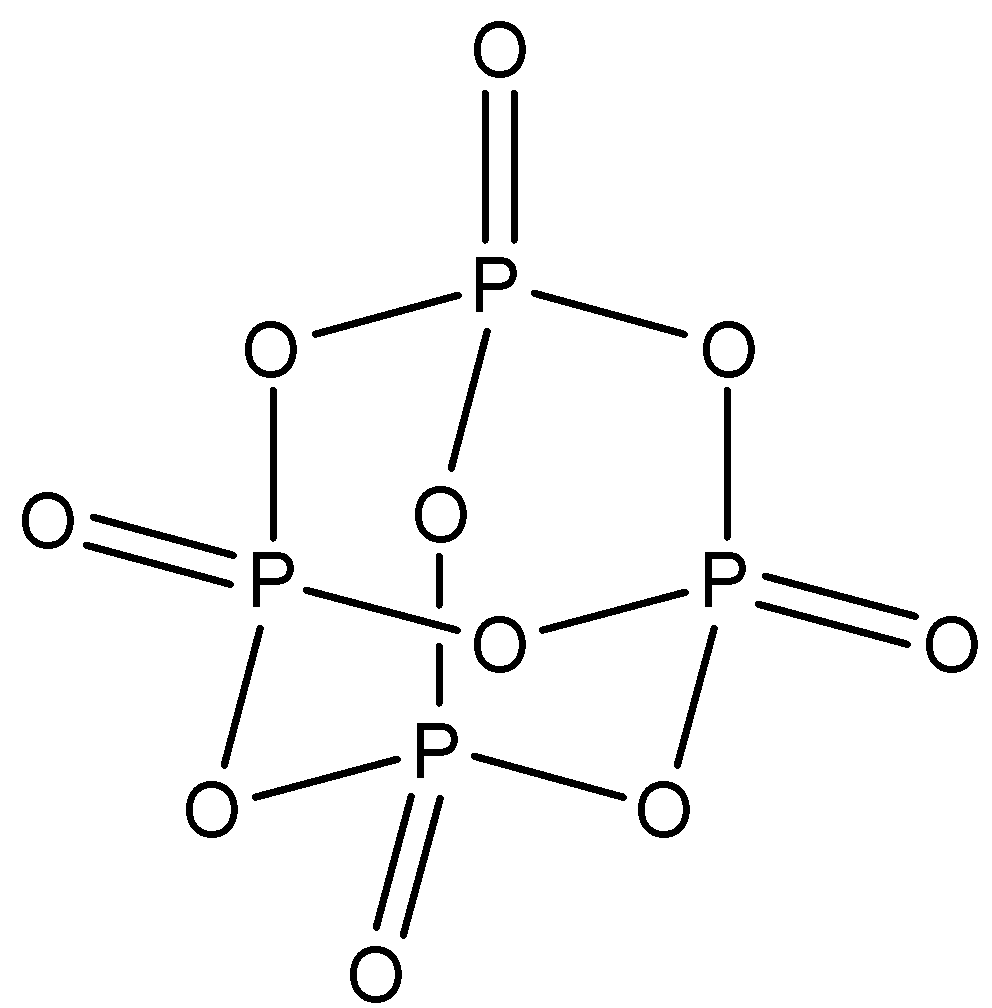
P4O10: This compound is known as phosphorus pentoxide. There are six P−O−P bonds in this structure and phosphorus is further bonded to one oxygen atom. The structure is:
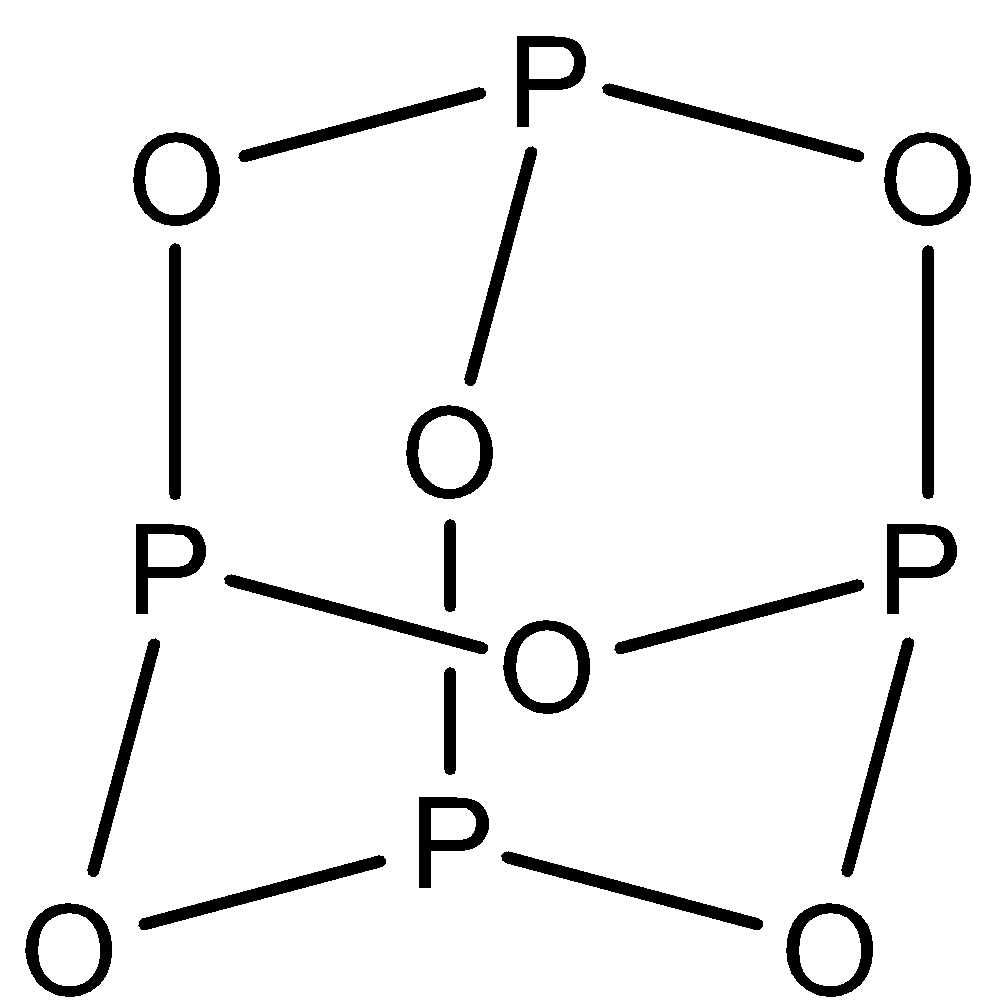
P4O6: This compound is known as phosphorus trioxide. There are six P−O−P bonds in this structure. The structure is:
H5P3O10: This compound is known as triphosphoric acid. This compound has three P−O−P bonds. The structure is given below:
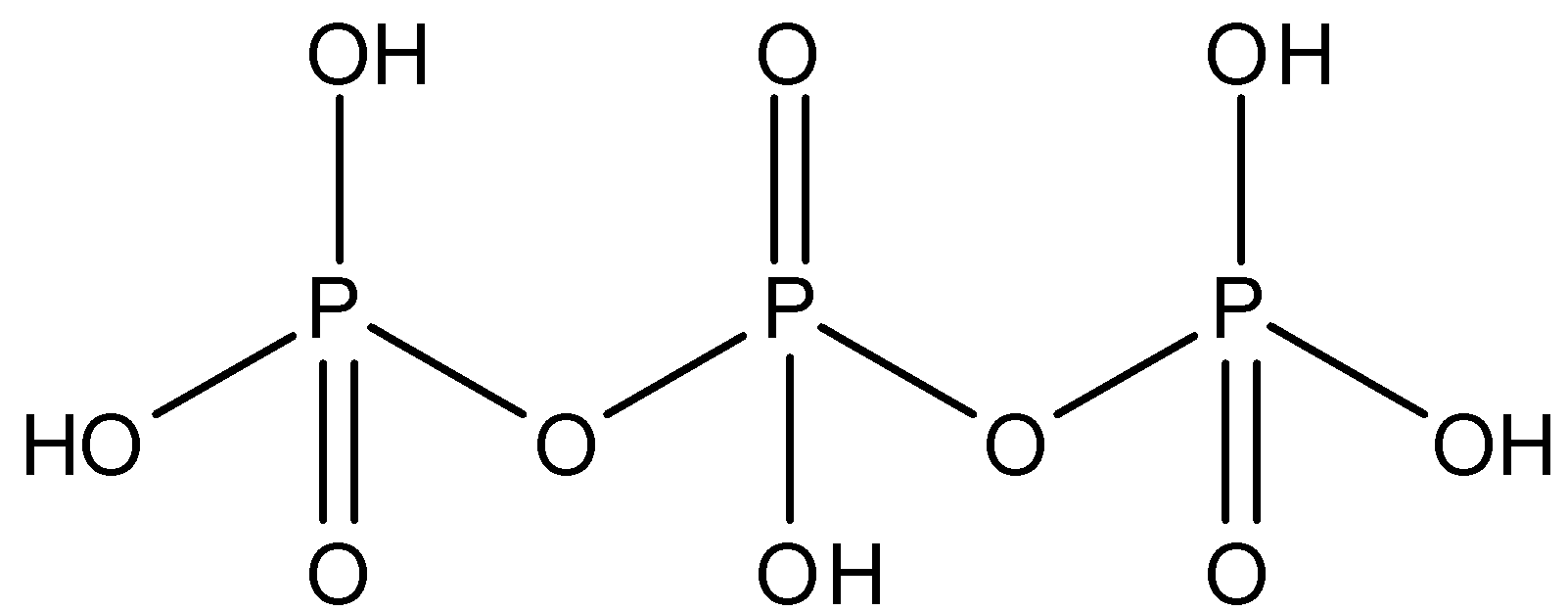
Si2O76−: This compound is known as disilicate. This compound has one Si−O−Si bond. The structure is given below:
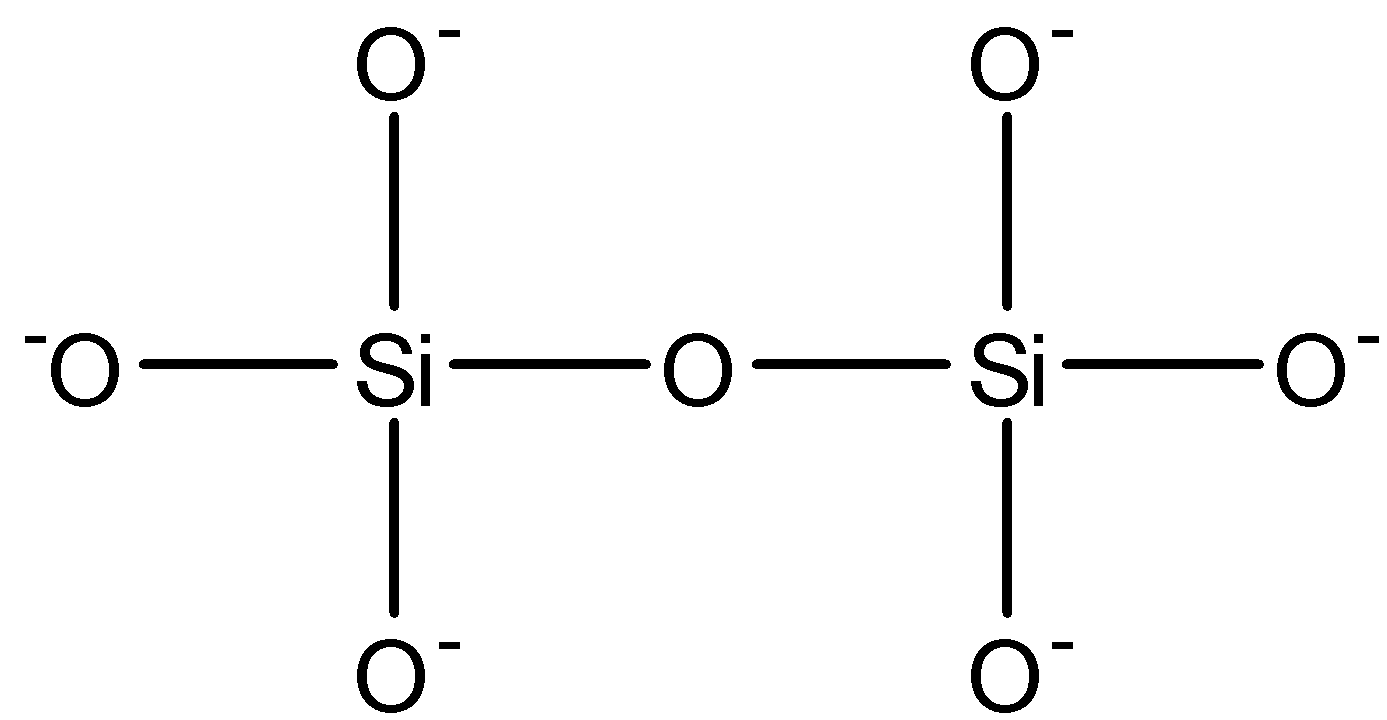
From the above discussion option (b) and option (d) are correct.
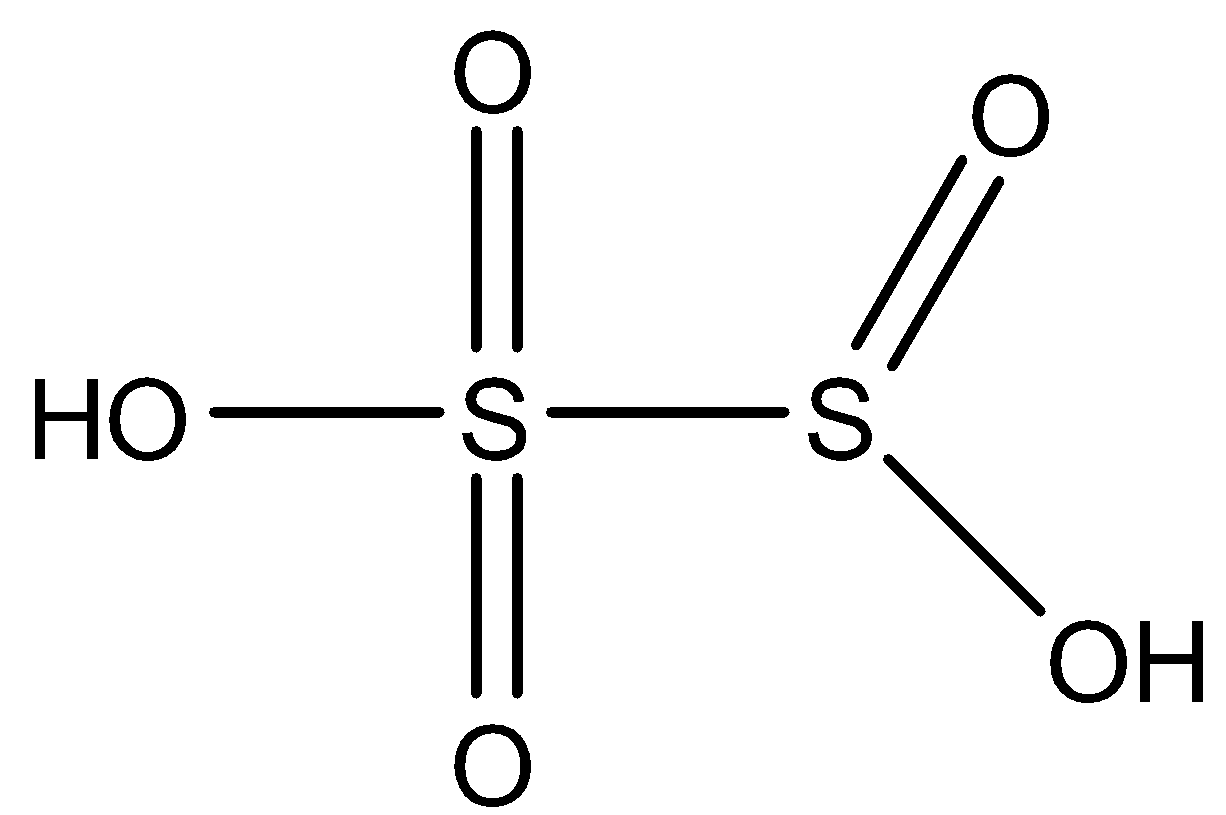
Note: All the oxoacids are mostly acidic. Other compounds that have a X−O−X bond is H2S2O7. This is known as Oleum and its structure is given below: