Question
Question: In which of the following molecules, the number of possible \[\angle XAS\] angles are maximum in the...
In which of the following molecules, the number of possible ∠XAS angles are maximum in the anionic part of their solid state? [A: Central atom, X: surrounding atom]
A. PBr5
B. N2O5
C. PCl5
D. Cl2O6
Solution
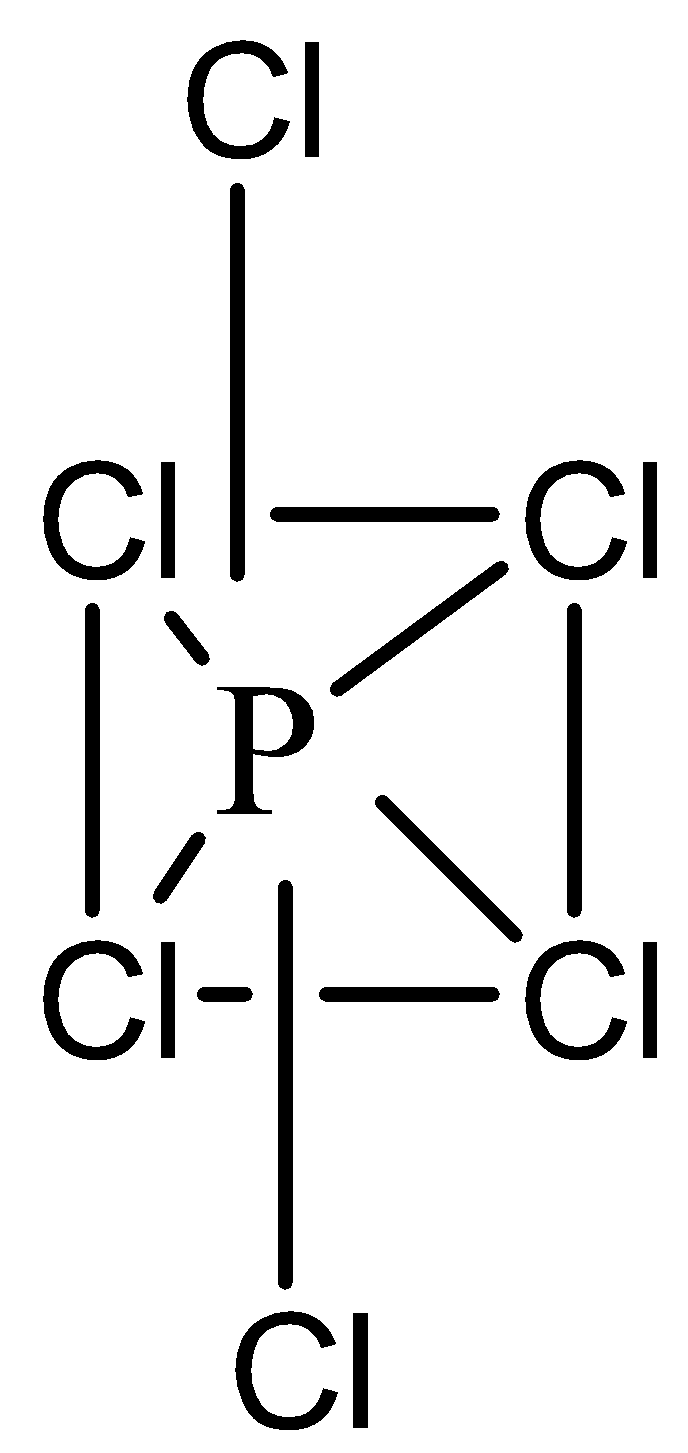
PCl5 molecule has trigonal bipyramidal geometry which is unstable as axial P−Cl bonds are slightly larger than equatorial P−Cl bonds. Also, some bond angles are of 90∘ and others are of 120∘. Due to this, trigonal bipyramidal geometry is not regular and not stable. Hence, in solid state, PCl5
molecule dissociates into tetrahedral [PCl4]+ and octahedral [PCl6]−
2PCl5(s)→[PCl4]++[PCl6]−
Complete step by step answer:
Actually PBr5 is known to exist in solid state as a highly reactive yellow solid. Solid PBr5 exists in the form of [PBr4]++[Br]−
PBr5(s)→[PBr4]++[Br]−
Both PCl5 and PBr5 trigonal bipyramidal geometry. This is not a regular structure and is not very stable. Therefore, PCl5 will split up into more stable octahedral and tetrahedral structures which are stable than trigonal bipyramidal.
2PCl5(s)→[PCl4]++[PCl6]−(sp3d2)
On the other hand, PBr5 splits up into stable tetrahedral structure as [PBr4]++[Br]−
PBr5(s)→[PBr4]++[Br]−
This splitting is different from PCl5 because Br atoms are large and six atoms of Br cannot be easily accommodated around smaller P atoms.
PCl5 has 2 angles of 90∘ and 3 angles of 120∘ (on the horizontal plane)
N2O5(s)→NO2++NO3−(sp2)
Cl2O6(s)→ClO2++ClO4−(sp3)
∠XAX angles are maximum in the anionic part of their solid-state is PCl5
Therefore, the correct answer is option (C).
Note: In solid state PCl5 tries to exist as oppositely charged ions like [PCl4]+ and [PCl6]− as the ionic bonding enhances the crystalline nature and also [PCl4]+ is tetrahedral , while PCl6−. is octahedral. These structures fit well into each other which gives more stability to solid structures.
