Question
Question: In which of the following molecules the central atom has three lone pairs of electrons? A) Ammonia...
In which of the following molecules the central atom has three lone pairs of electrons?
A) Ammonia.
B) Xenon difluoride.
C) Chlorine trifluoride.
D) Hydrogen sulphide.
Solution
We can calculate the steric number by totaling the number of atoms bonded to the central atom and the lone pairs of electrons present on the central metal atom. Let us know that if the steric number is 4, then we say the atom has sp3 hybridization, if the steric number is 3, then atom has sp2 hybridization, if the steric number is 2, then it has sp hybridization. We can also predict the geometry of compounds using the steric number.
Complete answer:
Let us see the structure of species in options,
The Lewis structure of xenon difluoride is,
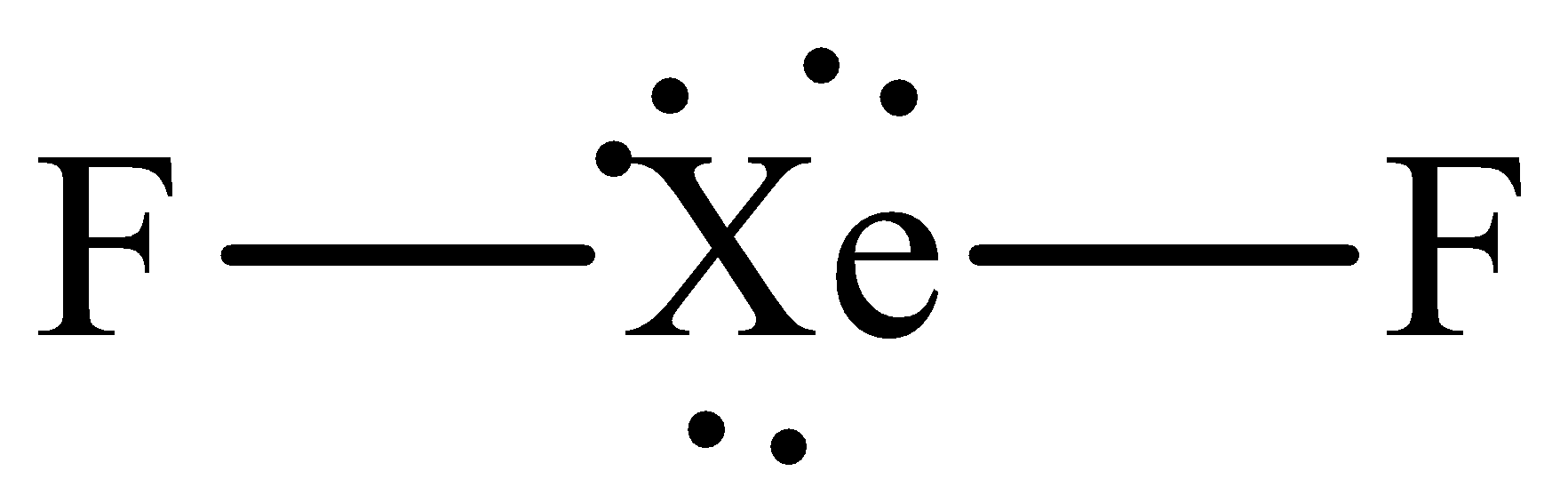
The steric number of central xenon atom is five which means that it is sp3d hybridized but it has only two bonding electrons thus it adopts the linear geometry with the bond angle of 180o .
Hence option B is correct.
The structure of NH3 is,
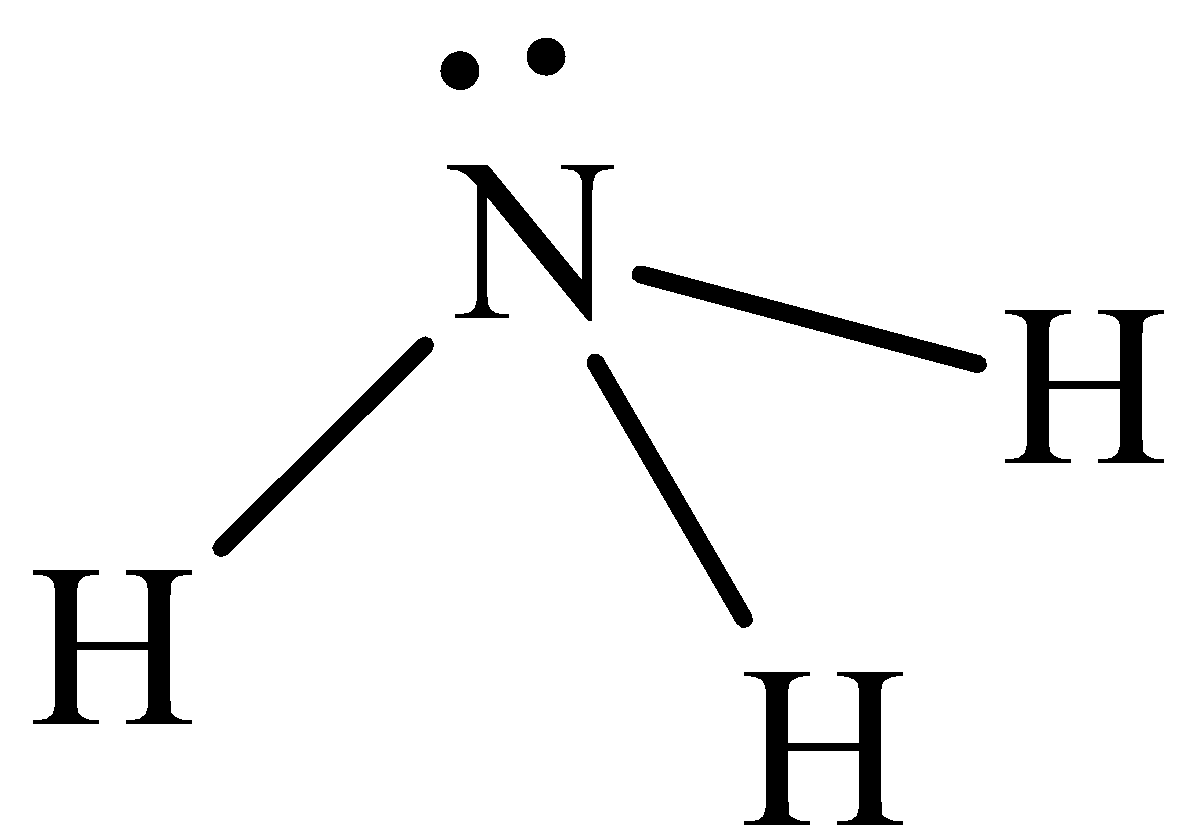
A NH3 molecule has three covalent bonds and one lone pair of electrons. Therefore, it is appropriate to use sp3 hybrid orbitals on the nitrogen atom. Three of these sp3 orbitals form localized bond orbitals by combining with the fluorine p orbitals. Thus, the bonding in a NH3 molecule in terms of three localized s-bond orbitals and one non-bonded lone pair in an sp3 orbital on the nitrogen atom. There are eight valence electrons in a NH3 molecule. Six of them occupy the three localized N (sp3) + (p) s-bond orbitals and two occupy the non-bonded N (sp3) orbital. The use of sp3 orbitals implies that the H–N–H bond angles are 109.5o . Hence option A is incorrect.
The structure of chlorine trifluoride is,
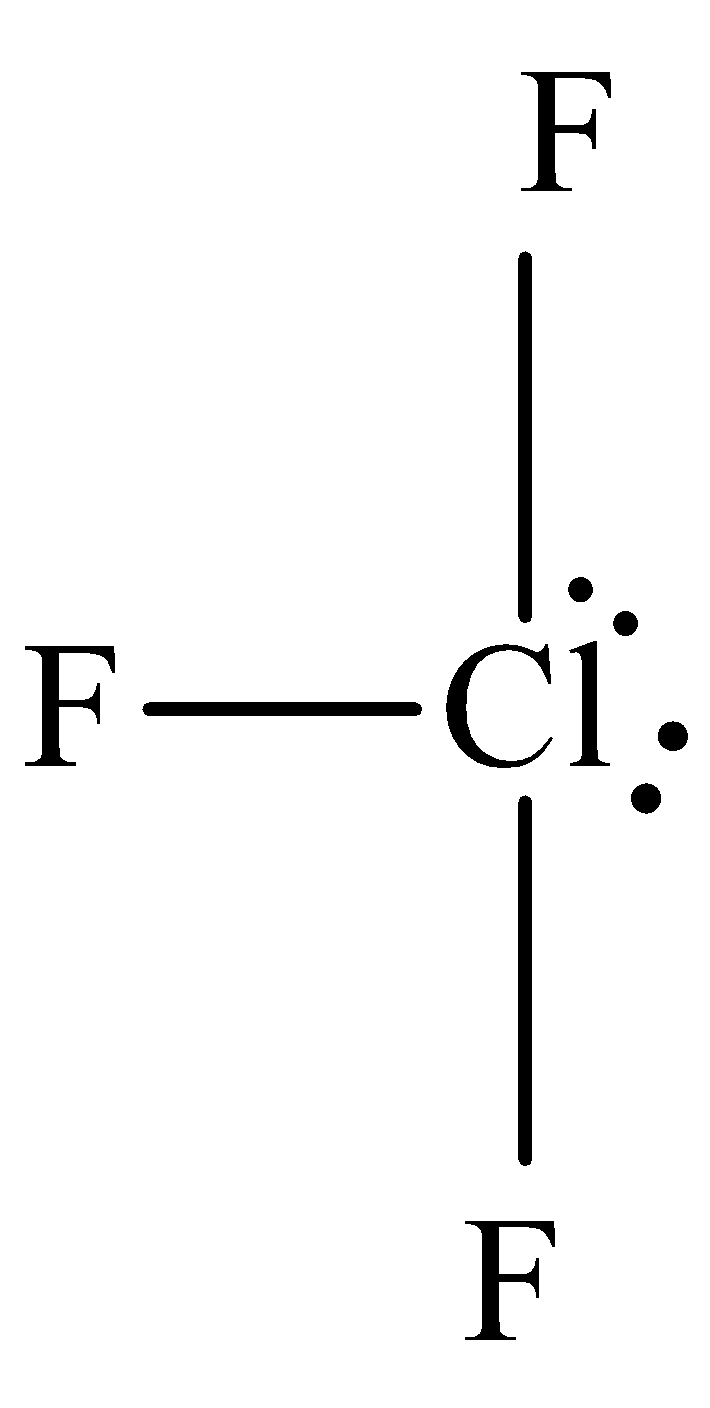
It is known that there are two lone pairs around the chlorine and thus option C is incorrect.
The structure of Hydrogen sulphide is,
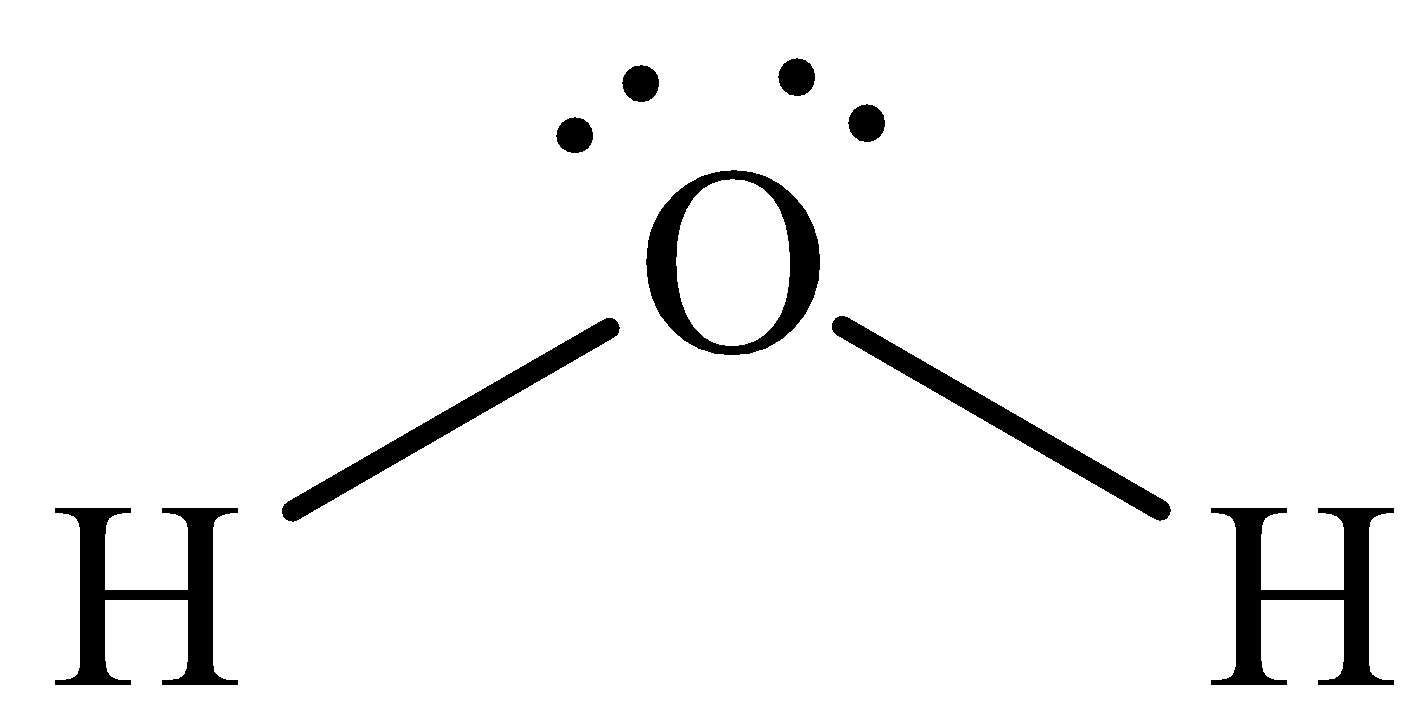
It is known that there are two lone pairs around the oxygen and thus option D is incorrect.
And hence option B is correct.
Note:
We have to remember that hybridization means the mixing of two atomic orbitals of the same energy to give a new hybrid molecular orbital of the same energy. Based on the type of orbitals involved, hybridization is classified as sp , sp2 , sp3 , dsp2 , dsp3 etc. Hybridization is often considered to have taken place with the assistance of an empty orbital. B contains 3 valence electrons, whereas 4 orbitals participate within the hybridization. Thus, one empty orbital participates within the hybridization.
