Question
Question: In which of the following molecules all atoms are not coplanar? * A. 
-
B.
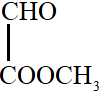
-
C.
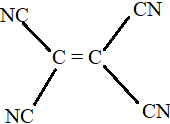
-
D.
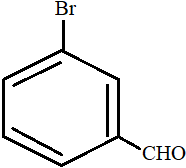
Solution
Hint- In order to deal with this question we will use the basic concept of organic chemistry which states that when a carbon atom is sp2 or sp hybridized, all the atoms attached to it are in a plane. In this question we will proceed further by understanding when carbon is said to be sp/sp2/sp3.
Complete answer:
sp : The third possible arrangement for carbon is sp hybridization which occurs when carbon is bound to two other atoms (two double bonds or one single + one triple bond).
sp2 : carbon is said to be sp2 hybridized when it is DOUBLY BONDED with any 1 atom and SINGLY BONDED with any other 2 atoms.
sp3 : The term “ sp3 hybridization” refers to the mixing character of one 2s-orbitals and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be sp3 hybridized, it must have an s orbital and three p orbitals
We know that when a carbon atom is sp2 or sp hybridized, all the atoms attached to it are in a plane.
All the carbon atoms in compounds A, C and D are sp2 hybridized. Hence, in these molecules, all the atoms are coplanar.
In option B, one carbon atom is sp3 hybridized. It has tetrahedral geometry. Hence, in this molecule, all atoms are not coplanar.
So, the correct answer is option B.
Note- Coplanar means atoms or groups of atoms that lie on the same plane. Like biphenyl, it has both benzene rings on the same plane. When all atoms of a compound are in the same plane are called coplanar compounds. Co planarity in organic compounds is seen in unsaturated molecules.
