Question
Question: In which of the following is the oxidation number fractional? (This question has multiple correct ...
In which of the following is the oxidation number fractional?
(This question has multiple correct options)
(A) B4H10
(B) B2O3
(C) C5O2
(D) KO3
Solution
Oxidation number of an atom is the total number of electrons that an atom either gains or losses in order to form a chemical bond with another atom. The oxidation number of an unknown atom in a molecule can be found if the oxidation numbers of the rest atoms are known.
Complete Solution :
So in the question four compounds are given and we have to find which of the compounds have fractional oxidation number. In the question itself it is given that there are multiple correct options for this question.
- We know that, the oxidation number or oxidation state of an atom describes the degree of oxidation (loss of electrons) or the degree of reduction (gain of electrons) in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component.
- In the given question, we will need to investigate each option individually:
(A)- B4H10
The B4H10 is a covalent compound which has four B-H-B bridges, no B-B-B triple bridge, one B-B bond and two terminal BH2 groups. It is known as tetraborane. The structure of tetraborane is:
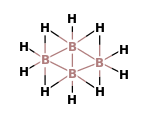
Now, we can calculate the oxidation number of B in this compound.
We know that there are four Boron atoms and ten oxide ions. Oxide ion possess a -2 charge hence we can find the oxidation state of B as,
4x+10(−2)=0
The oxidation state of a compound is zero.
4x+(−20)=0
⇒ 4x=20 x=420=5
⇒ x=420=+5
The oxidation state of B is +5.
(B) B2O3
B2O3 is a covalent compound which is a white, glassy solid. It is known as boron trioxide. The structure of boron trioxide is:
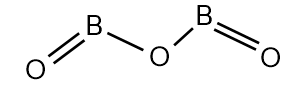
Let’s calculate the oxidation number of B in B2O3. There is two B atoms and three oxide ions inB2O3
⇒ 2x+3(−2)=0
⇒ 2x+(−6)=0
⇒ 2x=6
⇒ x=26=+3
Hence inB2O3, B is having an oxidation state of +3.
(C) C5O2
The compound C5O2 is known as pentacarbon dioxide. It is stable at room temperature in solution. The structure of pentacarbon dioxide is:
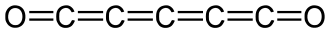
Let’s calculate the oxidation state of C in C5O2. There are five carbon atoms and two oxide ions.
Hence oxidation number is,
5x+2(−2)=0
⇒ 5x+(−4)=0
⇒ 5x=+4
⇒ x=5+4
Here the C is having a fraction as an oxidation number. Hence this is one of the correct answers for the question.
(D) KO3
KO3 is an ionic compound. The potassium always has a +1 oxidation state. The oxidation state of oxygen can be found.
⇒ 1+3x=0
⇒ 3x=−1
⇒ x=3−1
Here also the oxidation number obtained is a fractional number.
So, the correct answer is “Option C and D”.
Note: We should know the oxidation number of at least one atom then only we could calculate the oxidation state of the unknown, it is necessary to know the oxidation states of common atoms.
While calculating the oxidation states the sign should be always taken into consideration.
- Some students mix up valency and oxidation number, valency is a fixed number but oxidation number varies and in valency the sign does not have significance but it’s important in oxidation number.
